
a)
Interpretation:
The change has to be explained when -CN concentration is halved, and the 1-iodo-2-methylbutane concentration is doubled and Both the -CN and the 1-iodo-2-methylbutane concentrations are tripled.
Concept introduction:
SN2 reaction:
The alcohol is reaction with acids like hydrochloric acid or hydrobromic acid, the bromine atom attacks back side of the carbon atoms in simultaneous manner and which is bearing alcohol group which yield the corresponding product.
Example:
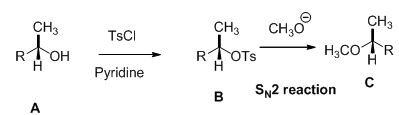
Alcohol is reaction with tosyl chloride in pyridine which provides retention of configuration of tosylated compound. This tosylated compound is further reaction with sodium methoxide which undergoes again SN2 type of reaction, the methoxide ion attacks the carbon atom through the back side and provides Inverse configuration of methoxy compound. This is shown below,
SN2 reaction is second order
b)
Interpretation:
The change has to be explained when -CN concentration is halved, and the 1-iodo-2-methylbutane concentration is doubled and Both the -CN and the 1-iodo-2-methylbutane concentrations are tripled.
Concept introduction:
SN2 reaction:
The alcohol is reaction with acids like hydrochloric acid or hydrobromic acid, the bromine atom attacks back side of the carbon atoms in simultaneous manner and which is bearing alcohol group which yield the corresponding product.
Example:
Alcohol is reaction with tosyl chloride in pyridine which provides retention of configuration of tosylated compound. This tosylated compound is further reaction with sodium methoxide which undergoes again SN2 type of reaction, the methoxide ion attacks the carbon atom through the back side and provides Inverse configuration of methoxy compound. This is shown below,

SN2 reaction is second order reaction, the rate of the reaction is depending on the both substrate and nucleophiles.
Trending nowThis is a popular solution!

Chapter 11 Solutions
OWLv2 with Student Solutions Manual eBook, 4 terms (24 months) Printed Access Card for McMurry's Organic Chemistry, 9th
- You are a Quality Manager for a very well-known food ingredient company that produces umami powder, and you are responsible for setting specification limits. The net weight (in grams) of bags of unami powder is monitored by taking samples of six bags on an hourly basis during production. The label on every bag reports a contents of 1KG umami powder. The process mean is μ = 1012 g, and when the process is properly adjusted, it varies with σ = 11 g. QUESTION: Using all the available information, set the upper and lower specification limits.arrow_forward43) 10.00 ml of vinegar (active ingredient is acetic acid) is titrated to the endpoint using 19.32 ml of 0.250 M sodium hydroxide. What is the molarity of acetic acid in the vinegar? YOU MUST SHOW YOUR WORK. NOTE: MA x VA = MB x VBarrow_forward424 Repon Sheet Rates of Chemical Reactions : Rate and Order of 1,0, Deception B. Effect of Temperature BATH TEMPERATURE 35'c Yol of Oh نام Time 485 Buret rend ing(n) 12 194 16. 6 18 20 10 22 24 14 115 95 14738 2158235 8:26 CMS 40148 Total volume of 0, collected Barometric pressure 770-572 ml mm Hg Vapor pressure of water at bath temperature (see Appendix L) 42.2 Slope Compared with the rate found for solution 1, there is Using the ideal gas law, calculate the moles of O; collected (show calculations) times faster 10 Based on the moles of O, evolved, calculate the molar concentration of the original 3% 1,0, solution (sho calculations)arrow_forward
- Steps and explanations pleasearrow_forwardUse diagram to answer the following: 1.Is the overall rxn endo- or exothermic. Explain briefly your answer____________________2. How many steps in this mechanism?_____________3. Which is the rate determining step? Explain briefly your answer____________________4. Identify (circle and label) the reactants,the products and intermediate (Is a Cation, Anion, or a Radical?) Please explain and provide full understanding.arrow_forwardDraw the entire mechanism and add Curved Arrows to show clearly how electrons areredistributed in the process. Please explain and provide steps clearly.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

