
(a)
Interpretation:
To predict the product of acid-catalyzed hydrolysis of given ester.
Concept Introduction:
An acid-catalyzed hydrolysis of the ester is a much faster reaction as compared to uncatalyzed hydrolysis of the ester. The addition of acid promotes the protonation of oxygen atom in the carbonyl group and as it is a fact that an oxygen atom with positive charge has more electron withdrawing tendency than neutral atom. The more withdrawal of electron density of oxygen atom decreases the electron density from the carbonyl carbon and make it more susceptible for the attack of the nucleophile. The acid catalyzed reaction mechanism is written as,
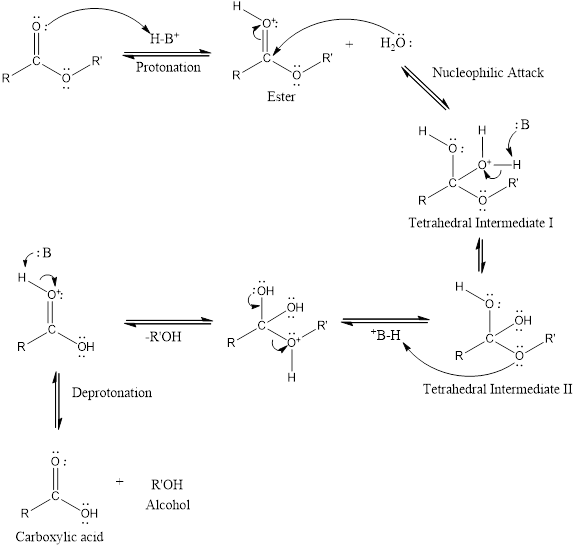
Therefore, products obtained by the acid catalyzed ester hydrolysis are the
(b)
Interpretation:
To predict the product of acid-catalyzed hydrolysis of given ester.
Concept introduction:
An acid-catalyzed hydrolysis of the ester is a much faster reaction as compared to uncatalyzed hydrolysis of the ester. The addition of acid promotes the protonation of oxygen atom in the carbonyl group and as it is a fact that an oxygen atom with positive charge has more electron withdrawing tendency than neutral atom. The more withdrawal of electron density of oxygen atom decreases the electron density from the carbonyl carbon and make it more susceptible for the attack of the nucleophile. The acid catalyzed reaction mechanism is written as,
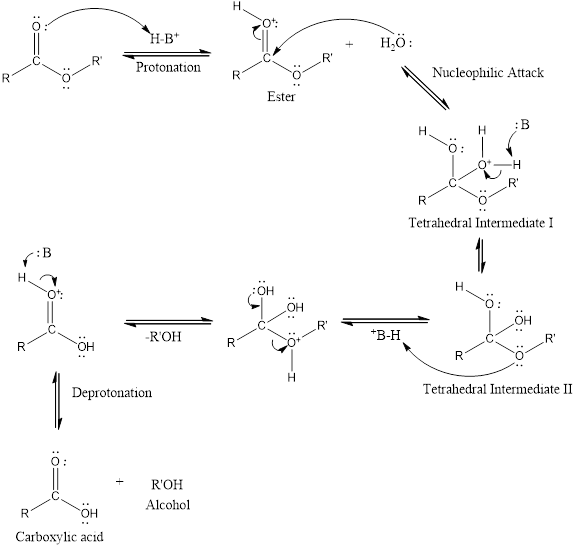
Therefore, products obtained by the acid catalyzed ester hydrolysis are the carboxylic acid and an alcohol by which ester was formed.
(c)
Interpretation:
To predict the product of acid-catalyzed hydrolysis of given ester.
Concept Introduction:
An acid-catalyzed hydrolysis of the ester is a much faster reaction as compared to uncatalyzed hydrolysis of the ester. The addition of acid promotes the protonation of oxygen atom in the carbonyl group and as it is a fact that an oxygen atom with positive charge has more electron withdrawing tendency than neutral atom. The more withdrawal of electron density of oxygen atom decreases the electron density from the carbonyl carbon and make it more susceptible for the attack of the nucleophile. The acid catalyzed reaction mechanism is written as,

Therefore, products obtained by the acid catalyzed ester hydrolysis are the carboxylic acid and an alcohol by which ester was formed.
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
Pearson eText for Essential Organic Chemistry -- Instant Access (Pearson+)
- Nonearrow_forwardPart II. Given below are the 'H-NMR spectrum at 300 MHz in CDC13 and mass spectrum using electron ionization of compound Brian. The FTIR of the said compound showed a strong peak at 1710 cm"). Determine the following: (a) molecular Formula and Degree of unsaturation of compound Brian (b) Basing on the given H-NMR spectrum tabulate the following (i) chemical shifts (ii) integration, ciii) multiplicity and (iv) interferences made for each signal (c) Draw the structure of compound Brian. ) ΕΙ 43 41 27 71 114 (M+) Hmmm 20 30 40 50 60 70 80 90 100 110 120 1H NMR spectrum 300 MHz in CDCl3 2.0 alle 1.0arrow_forwardThe iron-iron carbide phase diagram. In the diagram, the letter L indicates that it is a liquid. Indicate what its components are. Temperature (°C) 1600 10 Composition (at% C) 15 25 1538°C -1493°C 8 1400 1200 1394°C y+L L 2500 1147°C y. Austenite 2.14 4.30 2000 1000 912°C y + Fe3C 800ㅏ 0.76 0.022 600 a, Ferrite a + Fe3C 400 0 (Fe) Composition (wt% C) 727°C 1500 Cementite (Fe3C) 1000 6 6.70 Temperature (°F)arrow_forward
- Part V. Choose which isomer would give the 1H-NMR spectrum below. Justify your reasoning by assigning important signals to the Corresponding protons of the correct molecule. A D on of of of H H 88 2 90 7.8 7.6 7.4 80 5 6 [ppm] 7.2 6.8 6.6 6.4 ō [ppm]arrow_forwardShow work with explanation. don't give Ai generated solutionarrow_forwardQ7. a. Draw the line-bond structure of the major product for the following reaction, if a reaction occurs, assume monohalogenation. b. Calculate the product ratios using the following information (hint: use the number of hydrogens in each category present to calculate the ratios). Chlorination: 1° Reactivity=1 2° Reactivity=4 Heat + Cl2 3° Reactivity=5arrow_forward
- Please correct answer and don't use hand rating and don't use Ai solutionarrow_forwardQ10: Alkane halogenation a. Give the name and structures of the five isomeric hexanes. Page 4 of 5 Chem 0310 Organic Chemistry 1 Recitations b. For each isomer, give all the free radical monochlorination and monobromination products that are structurally isomeric.arrow_forwardQ9. The insecticide DDT (in the box below) is useful in controlling mosquito populations and has low toxicity to humans, but is dangerous to birds and fish. Hoping to alleviate the dangers, little Johnny Whizbang, an aspiring chemist, proposes a new version of DDT ("Bromo-DDT") and shows his synthesis to his boss. Will Johnny Whizbang's synthesis work? Or will he be fired? Assume there is an excess of bromine and polybrominated products can be separated. Explain why. CH3 Br2, light CBR3 ok-ok Br Br Br Br CI "Bromo-DDT" CCl 3 DDT (dichlorodiphenyltrichloroethane) CIarrow_forward
- Differentiate the terms Monotectic, Eutectic, Eutectoid, Peritectic, Peritectoid.arrow_forwardQ5. Predict the organic product(s) for the following transformations. If no reaction will take place (or the reaction is not synthetically useful), write "N.R.". Determine what type of transition state is present for each reaction (think Hammond Postulate). I Br₂ CH3 F2, light CH3 Heat CH3 F₂ Heat Br2, light 12, light CH3 Cl2, lightarrow_forwarda. For the following indicated bonds, rank them in order of decreasing AH° for homolytic cleavage. Based on your answer, which bond would be most likely to break homolytically? (a) (c) H3C CH3 .CH3 CH3 CH3 (b) Page 1 of 5 Chem 0310 Organic Chemistry 1 Recitations b. Draw all the possible radical products for 2-methylbutane, and determine which bond is most likely to be broken.arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


