
Write a structure for each of the following
- a. N,N – dimethylhexanamide
- b. 3,3 – dimethylhexanamide
- c. propionamide
- d. sodium acetate
- e. butyric anhydride
- f. 3 – methylbutanenitrile
(a)
Interpretation:
The strucutre for the given name of the compound has to be drawn.
Concept introduction:
The structure of the organic compound and its name are closely related to each other. The structure of the compound can be drawn if the name is given and vice-versa. The name of an organic compounds tells about the number of carbons present in the compound which is necessary so as to draw the carbon skeleton in the structure. The suffix of the name of the compound provides information about the functional group present in the compound.
Answer to Problem 31P
The structure of
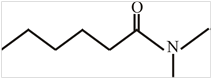
Explanation of Solution
The structure of the compound can be drawn if the name is given and vice-versa. The name of the compound is
Thus, the structure is drawn as,

(b)
Interpretation:
The strucutre for the given name of the compound has to be drawn.
Concept introduction:
The structure of the organic compound and its name are closely related to each other. The structure of the compound can be drawn if the name is given and vice-versa. The name of an organic compounds tells about the number of carbons present in the compound which is necessary so as to draw the carbon skeleton in the structure. The suffix of the name of the compound provides information about the functional group present in the compound.
Answer to Problem 31P
The structure of
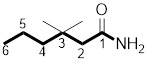
Explanation of Solution
The structure of the compound can be drawn if the name is given and vice-versa. The name of the compound is
Thus, the structure is drawn as,
(c)
Interpretation:
The strucutre for the given name of the compound has to be drawn.
Concept introduction:
The structure of the organic compound and its name are closely related to each other. The structure of the compound can be drawn if the name is given and vice-versa. The name of an organic compounds tells about the number of carbons present in the compound which is necessary so as to draw the carbon skeleton in the structure. The suffix of the name of the compound provides information about the functional group present in the compound.
Answer to Problem 31P
The structure of
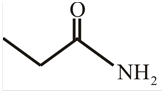
Explanation of Solution
The structure of the compound can be drawn if the name is given and vice-versa. The name of the compound is
Thus, the structure is drawn as,

(d)
Interpretation:
The strucutre for the given name of the compound has to be drawn.
Concept introduction:
The structure of the organic compound and its name are closely related to each other. The structure of the compound can be drawn if the name is given and vice-versa. The name of an organic compounds tells about the number of carbons present in the compound which is necessary so as to draw the carbon skeleton in the structure. The suffix of the name of the compound provides information about the functional group present in the compound.
Answer to Problem 31P
The structure of
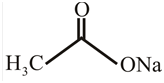
Explanation of Solution
The structure of the compound can be drawn if the name is given and vice-versa. The name of the compound is
Thus, the structure is drawn as,

(e)
Interpretation:
The strucutre for the given name of the compound has to be drawn.
Concept introduction:
The structure of the organic compound and its name are closely related to each other. The structure of the compound can be drawn if the name is given and vice-versa. The name of an organic compounds tells about the number of carbons present in the compound which is necessary so as to draw the carbon skeleton in the structure. The suffix of the name of the compound provides information about the functional group present in the compound.
Answer to Problem 31P
The structure of
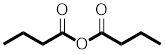
Explanation of Solution
The structure of the compound can be drawn if the name is given and vice-versa. The name of the compound is
The suffix is ‘anhydride’ indicating the presence of an acid anhydride group in which the
Thus, the structure is drawn as,

(f)
Interpretation:
The strucutre for the given name of the compound has to be drawn.
To draw the structure of the given compounds.
Concept introduction:
The structure of the organic compound and its name are closely related to each other. The structure of the compound can be drawn if the name is given and vice-versa. The name of an organic compounds tells about the number of carbons present in the compound which is necessary so as to draw the carbon skeleton in the structure. The suffix of the name of the compound provides information about the functional group present in the compound.
Answer to Problem 31P
The structure of 3-methylbutanenitrile is:
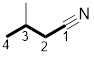
Explanation of Solution
The structure of the compound can be drawn if the name is given and vice-versa. The name of the compound is 3-methylbutanenitrile. It indicates that four carbons are present in the carbon skeleton of the compound. The suffix is ‘nitrile’ indicating the presence of a nitrile functional group. There is a methyl group on the third carbon in the chain. The numbering of carbons starts from the functional group side.
Thus, the structure is drawn as,

Want to see more full solutions like this?
Chapter 11 Solutions
Pearson eText for Essential Organic Chemistry -- Instant Access (Pearson+)
- we were assigned to dilute 900ppm in to 18ppm by using only 250ml vol flask. firstly we did calc and convert 900ppm to 0.9 ppm to dilute in 1 liter. to begin the experiment we took 0,225g of kmno4 and dissolved in to 250 vol flask. then further we took 10 ml sample sol and dissolved in to 100 ml vol flask and put it in to a spectrometer and got value of 0.145A . upon further calc we got v2 as 50ml . need to find DF, % error (expval and accptVal), molarity, molality. please write the whole report. thank you The format, tables, introduction, procedure and observation, result, calculations, discussion and conclusionarrow_forwardQ5. Predict the organic product(s) for the following transformations. If no reaction will take place (or the reaction is not synthetically useful), write "N.R.". Determine what type of transition state is present for each reaction (think Hammond Postulate). I Br₂ CH3 F2, light CH3 Heat CH3 F₂ Heat Br2, light 12, light CH3 Cl2, light Noarrow_forwardNonearrow_forward
- In the phase diagram of steel (two components Fe and C), region A is the gamma austenite solid and region B contains the gamma solid and liquid. Indicate the degrees of freedom that the fields A and B have,arrow_forwardFor a condensed binary system in equilibrium at constant pressure, indicate the maximum number of phases that can exist.arrow_forwardPart V. Label ad match the carbons in compounds Jane and Diane w/ the corresponding peak no. in the Spectra (Note: use the given peak no. To label the carbons, other peak no are intentionally omitted) 7 4 2 -0.13 -0.12 -0.11 -0.10 -0.08 8 CI Jane 1 -0.09 5 210 200 190 180 170 160 150 140 130 120 110 100 -8 90 f1 (ppm) 11 8 172.4 172.0 f1 (ppr HO CI NH Diane 7 3 11 80 80 -80 -R 70 60 60 2 5 -8 50 40 8. 170 160 150 140 130 120 110 100 90 -0 80 70 20 f1 (ppm) 15 30 -20 20 -60 60 -0.07 -0.06 -0.05 -0.04 -0.03 -0.02 -0.01 -0.00 -0.01 10 -0.17 16 15 56 16 -0.16 -0.15 -0.14 -0.13 -0.12 -0.11 -0.10 -0.09 -0.08 -0.07 -0.06 -0.05 -0.04 17.8 17.6 17.4 17.2 17.0 f1 (ppm) -0.03 -0.02 550 106 40 30 20 20 -0.01 -0.00 F-0.01 10 0arrow_forward
- n Feb 3 A T + 4. (2 pts) Draw the structure of the major component of the Limonene isolated. Explain how you confirmed the structure. 5. (2 pts) Draw the fragment corresponding to the base peak in the Mass spectrum of Limonene. 6. (1 pts) Predict the 1H NMR spectral data of R-Limonene. Proton NMR: 5.3 pon multiplet (H Ringarrow_forwardPart VI. Ca H 10 O is the molecular formula of compound Tom and gives the in the table below. Give a possible structure for compound Tom. 13C Signals summarized C1 C2 C3 C4 C5 C6 C7 13C shift (ppm) 23.5 27.0 33.0 35.8 127 162 205 DEPT-90 + DEPT-135 + +arrow_forward2. Using the following data to calculate the value of AvapH o of water at 298K. AvapH o of water at 373K is 40.7 kJ/mol; molar heat capacity of liquid water at constant pressure is 75.2J mol-1 K-1 and molar heat capacity of water vapor at constant pressure is 33.6 J mol-1 K-1.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





