
Pearson eText for Essential Organic Chemistry -- Instant Access (Pearson+)
3rd Edition
ISBN: 9780137533268
Author: Paula Bruice
Publisher: PEARSON+
expand_more
expand_more
format_list_bulleted
Question
Chapter 11, Problem 40P
(a)
Interpretation Introduction
Interpretation: The equation for the hydrolysis of propanoyl chloride has to be written.
Concept Introduction:
Hydrolysis of Acyl chloride:
Hydrolysis is the reaction of acyl chloride with water molecule. In this reaction, the acyl chloride gets converted into
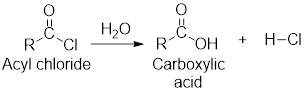
(b)
Interpretation Introduction
Interpretation: The equation for the transesterification of ethyl butanoate with propanol has to be written.
Concept Introduction:
Transesterification reaction:
- It an example for an alcoholysis reaction.
- The alcoholysis reaction is just like hydrolysis in which one component gets converted into two components using a molecule of alcohol like using a molecule of water in hydrolysis.
- The alcoholysis of ester is known as transesterification reaction. In this reaction one ester component reacts with a molecule of alcohol and will be converted into two components such as another new ester and an alcohol which has been shown in the following general scheme:

(c)
Interpretation Introduction
Interpretation: The equation for the aminolysis of ethyl pentanoate has to be written.
Concept Introduction:
- The aminolysis reaction is just like hydrolysis in which one component gets converted into two components using a molecule of
amine like using a molecule of water in hydrolysis. - In the aminolysis of ester, one ester component reacts with a molecule of amine and will be converted into two components such as an amide and an alcohol which has been shown in the following general scheme:
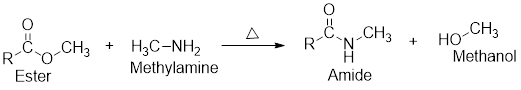
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
1) Suppose 0.1 kg ice at 0°C (273K) is in 0.5kg water at 20°C (293K). What is the change in entropy of the ice as it melts at 0°?
To produce the original "water gas" mixture, carbon (in a combustible form known as coke) is reacted with steam: 131.4 kJ + H20(g) + C(s) → CO(g) + H2(g) From this information and the equations in the previous problem, calculate the enthalpy for the combustion or carbon to form carbon dioxide.
kindly show me how to solve both parts of the same long problem. Thanks
we were assigned to dilute 900ppm
in to 18ppm by using only 250ml vol
flask. firstly we did calc and convert
900ppm to 0.9 ppm to dilute in 1 liter.
to begin the experiment we took
0,225g of kmno4 and dissolved in to
250 vol flask. then further we took 10
ml sample sol and dissolved in to 100
ml vol flask and put it in to a
spectrometer and got value of 0.145A
.
upon further calc we got v2 as 50ml
. need to find DF, % error (expval and
accptVal), molarity, molality. please
write the whole report. thank you
The format, tables, introduction,
procedure and observation, result,
calculations, discussion and
conclusion
Q5. Predict the organic product(s) for the following transformations. If no reaction will take place
(or the reaction is not synthetically useful), write "N.R.". Determine what type of transition state
is present for each reaction (think Hammond Postulate).
I
Br₂
CH3
F2, light
CH3
Heat
CH3
F₂
Heat
Br2, light
12, light
CH3
Cl2, light
No
Chapter 11 Solutions
Pearson eText for Essential Organic Chemistry -- Instant Access (Pearson+)
Ch. 11.1 - The aromas of many flowers and fruits are due to...Ch. 11.1 - Name the following compounds:Ch. 11.1 - Prob. 3PCh. 11.2 - Prob. 4PCh. 11.2 - Prob. 5PCh. 11.4 - a. What is the product of the reaction of acetyl...Ch. 11.4 - Prob. 7PCh. 11.5 - Using the pKa values listed in Table 11.1, predict...Ch. 11.6 - Starting with acetyl chloride, what neutral...Ch. 11.6 - Prob. 10P
Ch. 11.7 - Prob. 11PCh. 11.8 - Prob. 13PCh. 11.8 - Using the mechanism for the acidcatalyzed...Ch. 11.8 - Prob. 15PCh. 11.8 - Prob. 16PCh. 11.8 - Prob. 17PCh. 11.9 - Prob. 18PCh. 11.10 - Show how each of the following esters could be...Ch. 11.11 - Which of the following reactions would lead to the...Ch. 11.12 - Prob. 22PCh. 11.12 - Prob. 23PCh. 11.13 - Prob. 24PCh. 11.13 - Prob. 25PCh. 11.14 - Prob. 26PCh. 11.14 - Prob. 27PCh. 11.14 - Prob. 28PCh. 11.15 - Prob. 29PCh. 11.15 - How would you synthesize the following compounds...Ch. 11 - Write a structure for each of the following a. N,N...Ch. 11 - Prob. 32PCh. 11 - Which ester is more reactive, methyl acetate or...Ch. 11 - What products would be formed from the reaction of...Ch. 11 - What products would be obtained from the following...Ch. 11 - Prob. 36PCh. 11 - a. Which compound would you expect to have a...Ch. 11 - a. List the following esters in order of...Ch. 11 - D. N. Kursanov, a Russian chemist, proved that the...Ch. 11 - Prob. 40PCh. 11 - Using an alcohol for one method and an alkyl...Ch. 11 - Prob. 42PCh. 11 - Prob. 44PCh. 11 - Prob. 45PCh. 11 - Prob. 46PCh. 11 - Prob. 47PCh. 11 - Prob. 48PCh. 11 - Prob. 49PCh. 11 - Show how the following compounds could be prepared...Ch. 11 - Prob. 51PCh. 11 - Prob. 52PCh. 11 - Prob. 53P
Knowledge Booster
Similar questions
- Nonearrow_forwardIn the phase diagram of steel (two components Fe and C), region A is the gamma austenite solid and region B contains the gamma solid and liquid. Indicate the degrees of freedom that the fields A and B have,arrow_forwardFor a condensed binary system in equilibrium at constant pressure, indicate the maximum number of phases that can exist.arrow_forward
- Part V. Label ad match the carbons in compounds Jane and Diane w/ the corresponding peak no. in the Spectra (Note: use the given peak no. To label the carbons, other peak no are intentionally omitted) 7 4 2 -0.13 -0.12 -0.11 -0.10 -0.08 8 CI Jane 1 -0.09 5 210 200 190 180 170 160 150 140 130 120 110 100 -8 90 f1 (ppm) 11 8 172.4 172.0 f1 (ppr HO CI NH Diane 7 3 11 80 80 -80 -R 70 60 60 2 5 -8 50 40 8. 170 160 150 140 130 120 110 100 90 -0 80 70 20 f1 (ppm) 15 30 -20 20 -60 60 -0.07 -0.06 -0.05 -0.04 -0.03 -0.02 -0.01 -0.00 -0.01 10 -0.17 16 15 56 16 -0.16 -0.15 -0.14 -0.13 -0.12 -0.11 -0.10 -0.09 -0.08 -0.07 -0.06 -0.05 -0.04 17.8 17.6 17.4 17.2 17.0 f1 (ppm) -0.03 -0.02 550 106 40 30 20 20 -0.01 -0.00 F-0.01 10 0arrow_forwardConsider the reaction of 2-methylpropane with a halogen. With which halogen will the product be almost exclusively 2-halo-2-methylpropane? 1. F2 2. Cl2 3. Br2 4. I2arrow_forwardNonearrow_forward
- Nonearrow_forwardn Feb 3 A T + 4. (2 pts) Draw the structure of the major component of the Limonene isolated. Explain how you confirmed the structure. 5. (2 pts) Draw the fragment corresponding to the base peak in the Mass spectrum of Limonene. 6. (1 pts) Predict the 1H NMR spectral data of R-Limonene. Proton NMR: 5.3 pon multiplet (H Ringarrow_forwardPart VI. Ca H 10 O is the molecular formula of compound Tom and gives the in the table below. Give a possible structure for compound Tom. 13C Signals summarized C1 C2 C3 C4 C5 C6 C7 13C shift (ppm) 23.5 27.0 33.0 35.8 127 162 205 DEPT-90 + DEPT-135 + +arrow_forward
- 2. Using the following data to calculate the value of AvapH o of water at 298K. AvapH o of water at 373K is 40.7 kJ/mol; molar heat capacity of liquid water at constant pressure is 75.2J mol-1 K-1 and molar heat capacity of water vapor at constant pressure is 33.6 J mol-1 K-1.arrow_forwardPart VII. Below are the 'HNMR 13 3 C-NMR, COSY 2D- NMR, and HSQC 20-NMR (Similar with HETCOR but axes are reversed) spectra of an organic compound with molecular formula C6H13 O. Assign chemical shift values to the H and c atoms of the compound. Find the structure. Show complete solutions. Predicted 1H NMR Spectrum ли 4.7 4.6 4.5 4.4 4.3 4.2 4.1 4.0 3.9 3.8 3.7 3.6 3.5 3.4 3.3 3.2 3.1 3.0 2.9 2.8 2.7 2.6 2.5 2.4 2.3 2.2 2.1 2.0 1.9 1.8 1.7 1.6 1.5 1.4 1.3 1.2 1.1 1.0 0.9 0.8 f1 (ppm)arrow_forward3. Draw the expanded structural formula, the condensed structural formula, and the skeletal structural formula for 2-pentene. expanded structure: Condensed structure: Skeletal formula: 4. Draw the expanded structural formula, the condensed structural formula, and the skeletal structural formula for 2-methyl-3-heptene. expanded structure: Condensed structure: Skeletal formula: following structurearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning