
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
2nd Edition
ISBN: 9780077633707
Author: Janice Smith
Publisher: Mcgraw-hill Higher Education (us)
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 11.3, Problem 11.8P
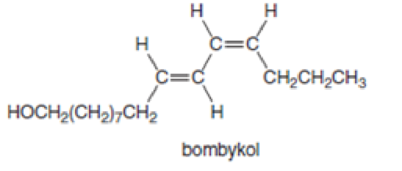
Bombykol is secreted by the female silkworm moth (Bombyx mori) to attract mates. Bombykol contains two double bonds, and each double bond must have a particular three-dimensional arrangement of groups around it to be biologically active. Label the double bonds of bombykol as cis or trans.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Stereoisomers share the same connectivity and differ only in the way their atoms are arranged in space. Draw the structure of a compound that is a stereoisomer of
Gg.194.
Drawn are four isomeric dimethylcyclopropanes.
Would an equal mixture of compounds C and D be optically active?What about an equal mixture of B and C?
Chapter 11 Solutions
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
Ch. 11.1 - Convert each condensed structure to a complete...Ch. 11.1 - Determine whether each molecular formula...Ch. 11.1 - Give the molecular formula for each of the...Ch. 11.2 - Give the IUPAC name for each alkene. a. (CH3CH2)2C...Ch. 11.2 - Prob. 11.5PCh. 11.2 - Give the structure corresponding to each name. a....Ch. 11.3 - Prob. 11.7PCh. 11.3 - Bombykol is secreted by the female silkworm moth...Ch. 11.3 - Prob. 11.9PCh. 11.3 - Prob. 11.10P
Ch. 11.3 - Prob. 11.11PCh. 11.5 - Prob. 11.12PCh. 11.5 - Prob. 11.13PCh. 11.6 - Prob. 11.14PCh. 11.6 - Prob. 11.15PCh. 11.7 - Prob. 11.16PCh. 11.7 - Prob. 11.17PCh. 11.9 - Prob. 11.18PCh. 11.9 - Draw the structure corresponding to each name. a....Ch. 11.10 - Prob. 11.20PCh. 11.10 - Prob. 11.21PCh. 11.10 - Prob. 11.22PCh. 11 - Prob. 11.23UKCCh. 11 - Prob. 11.24UKCCh. 11 - Prob. 11.25UKCCh. 11 - Prob. 11.26UKCCh. 11 - Answer the following questions about compound A,...Ch. 11 - Prob. 11.28UKCCh. 11 - Prob. 11.29UKCCh. 11 - Prob. 11.30UKCCh. 11 - Prob. 11.31UKCCh. 11 - Prob. 11.32UKCCh. 11 - Prob. 11.33APCh. 11 - Prob. 11.34APCh. 11 - Prob. 11.35APCh. 11 - Prob. 11.36APCh. 11 - Prob. 11.37APCh. 11 - Falcarinol is a natural pesticide found in carrots...Ch. 11 - Prob. 11.39APCh. 11 - Prob. 11.40APCh. 11 - Prob. 11.41APCh. 11 - Prob. 11.42APCh. 11 - Prob. 11.43APCh. 11 - Give the structure corresponding to each IUPAC...Ch. 11 - Leukotriene C4 is a key compound that causes the...Ch. 11 - Prob. 11.46APCh. 11 - Prob. 11.47APCh. 11 - Prob. 11.48APCh. 11 - Prob. 11.49APCh. 11 - Prob. 11.50APCh. 11 - Prob. 11.51APCh. 11 - Prob. 11.52APCh. 11 - Prob. 11.53APCh. 11 - Prob. 11.54APCh. 11 - Prob. 11.55APCh. 11 - Prob. 11.56APCh. 11 - Prob. 11.57APCh. 11 - Draw the products formed in each reaction.Ch. 11 - Prob. 11.59APCh. 11 - Prob. 11.60APCh. 11 - Prob. 11.61APCh. 11 - Prob. 11.62APCh. 11 - Prob. 11.63APCh. 11 - Prob. 11.64APCh. 11 - Prob. 11.65APCh. 11 - Prob. 11.66APCh. 11 - Prob. 11.67APCh. 11 - Prob. 11.68APCh. 11 - Prob. 11.69APCh. 11 - Prob. 11.70APCh. 11 - Prob. 11.71APCh. 11 - Prob. 11.72APCh. 11 - Prob. 11.73APCh. 11 - Prob. 11.74APCh. 11 - Prob. 11.75APCh. 11 - Prob. 11.76APCh. 11 - Prob. 11.77APCh. 11 - Prob. 11.78APCh. 11 - Prob. 11.79APCh. 11 - Prob. 11.80APCh. 11 - Prob. 11.81APCh. 11 - Prob. 11.82APCh. 11 - Prob. 11.83APCh. 11 - Prob. 11.84APCh. 11 - Prob. 11.85APCh. 11 - Prob. 11.86APCh. 11 - Are cis-2-hexene and trans-3-hexene constitutional...Ch. 11 - Prob. 11.88CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- #23arrow_forwardb) C8H13Bг2Cl2O2 Isomer #1: Name of Isomer #1: Name of Isomer #2: Name of Isomer #3: Isomer #2: Isomer #3:arrow_forwardBromine is a larger atom than chlorine, but the equilibrium constants in Table 3.9 indicate that a chloro substituent has a greater preference for the equatorial position than does a bromo substituent. Suggest an explanation for this fact.arrow_forward
- 1. Draw the two conformations of trans-1-bromo-3-methylcyclohexane using both the Newman projection and the chair drawing. Calculate the energies of each. What ratio of the two conformers will be present at equilibrium? 2. Draw a structure of a chiral alcohol.arrow_forwardPyrethrin II and pyrethrosin are two natural products isolated from plants of the chrysanthemum family. Pyrethrin II is a natural insecticide and is marketed as such. Q,) Label all chiral centers in each molecule and all carbon-carbon double bonds about which there is the possibility for cis,trans isomerism.arrow_forwardClassify each description as a property of either alcohols, ethers, or both alcohols and ethers. Alcohols Ethers Both alcohols and ethers See attachment for the options for answersarrow_forward
- Give the IUPAC names and common names of the ff.arrow_forward6. Use the molecule below to answer the following questions. a) Name the following molecule. b) Why is it necessary to provide R or S designation in order to clearly communicate molecular structure for this molecule? c) How many stereoisomers of this molecule are possible? Which of them are enantiomers to each other? Which are diastereomers? SH ICNarrow_forwardClassifying a carbon atom by the number of carbons to which it is bonded can also be done in more complex molecules that contain heteroatoms. Classify each sp' hybridized carbon atom in bilobalide, a compound isolated from Ginkgo biloba extracts, as 1°, 2°, 3°, or 4°. Be sure to answer all parts. b НО -h a O: bilobalide а: b: c: d: e: f: g: Oarrow_forward
- Two VSEPR diagrams for Molecule 1 are given below. They are conformational isomers or molecules that place atoms differently in space because of the rotation of a single bond. H H H:O H:O Conformer 1 H H H Ö: Conformer 2 3. (a) Can you “see” either of the two conformational isomers (Conformer 1 or Conformer 2) in the line drawing that you drew for Molecule 1? Explain your answer.arrow_forwardThe shrub ma huang (Section 5.4A) contains two biologically active stereoisomers—ephedrine and pseudoephedrine—with two stereogenic centers as shown in the given structure. Ephedrine is one component of a once-popular combination drug used by body builders to increase energy and alertness, whereas pseudoephedrine is a nasal decongestant. a.Draw the structure of naturally occurring (−)-ephedrine, which has the 1R,2S configuration. b.Draw the structure of naturally occurring (+)-pseudoephedrine, which has the 1S,2S configuration. c.How are ephedrine and pseudoephedrine related? d.Draw all other stereoisomers of (−)-ephedrine and (+)pseudoephedrine, and give the R,S designation for all stereogenic centers. e.How is each compound drawn in part (d) related to (−)-ephedrine?arrow_forwardDraw a structural formula and Draw the structure of a compound that is a stereoisomerarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY