
Concept explainers
(a)
Interpretation:
Whether the given pair of compounds related as constitutional isomers, stereoisomers, or identical has to be indicated.
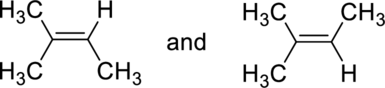
Concept Introduction:
Constitutional isomer is nothing but the structural isomer which has same molecular formula but differ in their connectivity of atoms.
Stereoisomers are spatial isomers which have same molecular formula and have differ in special arrangements which means the three-dimensional orientations of their atoms in space are arranged in different manner.
(b)
Interpretation:
Whether the given pair of compounds related as constitutional isomers, stereoisomers, or identical has to be indicated.
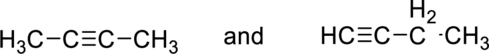
Concept Introduction:
Refer to part (a).
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
- Distinguish between isomerism and resonance. Distinguish between structural and geometric isomerism. When writing the various structural isomers, the most difficult task is identifying which are different isomers and which are identical to a previously written structurethat is, which are compounds that differ only by the rotation of a carbon single bond. How do you distinguish between structural isomers and those that are identical? Alkenes and cycloalkanes are structural isomers of each other. Give an example of each using C4H8. Another common feature of alkenes and cycloalkanes is that both have restricted rotation about one or more bonds in the compound, so both can exhibit cis- trans isomerism. What is required for an alkene or cycloalkane to exhibit cis-trans isomerism? Explain the difference between cis and trans isomers. Alcohols and ethers are structural isomers of each other, as are aldehydes and ketones. Give an example of each to illustrate. Which functional group in Table 21-4 can be structural isomers of carboxylic acids? What is optical isomerism? What do you look for to determine whether an organic compound exhibits optical isomerism? 1-Bromo-1-chloroethane is optically active whereas 1-bromo-2-chloroethane is not optically active. Explain.arrow_forwardIs it possible for a motor fuel to have a negative octane rating? Explain.arrow_forwardPlease don't provide handwriting solutionarrow_forward
- Draw the structure of all compounds that fit the following descriptions. a. Five constitutional isomers having the molecular formula C4Hg.arrow_forwardQuestion 40 of 67 Identify the relationship between these two compounds. HO H CH₂CH3 H H CH3 A) enantiomers B) diastereomers HO H C) conformational isomers D) constitutional isomers H CH3 CH₂CH3arrow_forwardHW24arrow_forward
- The octane rating for gasoline is a measurement of how readily a fuelcombusts compared to 2,2,4-trimethylpentane, an isomer of octane.a. Draw 2,2,4-trimethylpentane and verify that it is an isomer ofoctane.b. Draw four other isomers of octane.c. Select one of the isomers and draw it such that it looksdifferent on the page but is still the exact same compound.d. Name this isomer.e. Define isomer using a complete sentence.arrow_forwardQuestion 7 of 7 Identify each pair of compounds as constitutional isomers, stereoisomers, identical molecules, or other. O Macmillan Learning > compound A CH3 compound C CH3 compound B CH3 CH3 compound D Compounds A and B are constitutional isomers. not related. stereoisomers. identical molecules. Compounds C and D are stereoisomers. not related. identical molecules. constitutional isomers.arrow_forward3. occur when molecules change shape due to the rotation of atoms around single bonds. Conformations Stereoisomers Structural isomers Positional isomersarrow_forward
- ISOMERISMarrow_forwardCompounds that can cause cancer is called carcinogens. Many pesticides are carcinogenic. Based on your knowledge of organic chemistry, what could be the reason behind the carcinogenicity of pesticides? O a. Due to the presence of C-X bond O b. Due to the low boiling point of pesticides Due to the presence of C-C double bond O d. Due to stereoisomerism posses by pesticidesarrow_forward1. a. Draw and name the five cycloalkane structures of formula C5H10. Can any of these structures give rise to geometric (cis-trans) isomerism? If so, show the cis and trans stereoisomers. b. Draw and name the eight cycloalkane structures of formula C6H12 that do not show geometric isomerism. c. Draw and name the four cycloalkanes of formula C6H12 that do have cis-trans isomers. 2. Each of the following descriptions applies to more than one alkane. In each case, draw and name two structures that match the description. (a) an isopropylheptane (b) a diethyldecane (c) a cis-diethylcyclohexane (d) a trans-dihalocyclopentane (e) a (2,3-dimethylpentyl)cycloalkane (f) a bicyclononane 3. 2. refer to the photo attached and answer the ff.3-33, 3-34arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





