
Concept explainers
(a)
Interpretation:
Which
Concept Introduction:
Hydaration reaction is nothing but the addition of water to an alkene. In this reaction two bonds are simultaneously broken, one is carbon-carbon double bond, and second one is
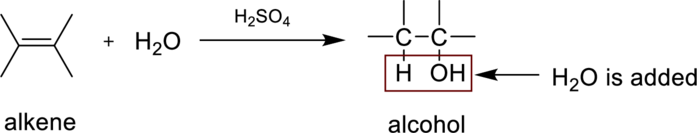
In the addition of water to an unsymmetrical alknene, the hydrogen from water is bonded to the less substituted carbon atom and is called Markovnikov’s rule.
(b)
Interpretation:
Which alkene as a starting material used to prepare 2-pentanol has to be determined.
Concept Introduction:
Hydaration reaction is nothing but the addition of water to an alkene. In this reaction two bonds are simultaneously broken, one is carbon-carbon double bond, and second one is
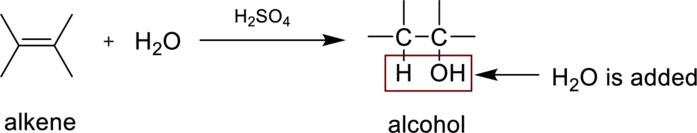
In the addition of water to an unsymmetrical alknene, the hydrogen from water is bonded to the less substituted carbon atom and is called Markovnikov’s rule.
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
- Ppplllleeeaaasssseeee helllppp wiithhh thisss Organic chemistryyyyyy I talked like this because AI is very annoyingarrow_forwardName the family to which each organic compound belongs. The first answer has been filled in for you. compound CH₂ || CH3-C-NH2 0 ။ CH3-C-CH₂ CH=O–CH=CH, CH₂ HO CH2-CH2-CH-CH3 family amine Darrow_forward1b. Br LOHarrow_forward
- I would like my graphs checked please. Do they look right? Do I have iodine and persulfate on the right axis ?arrow_forwardReaction Fill-ins Part 2! Predict the product(s) OR starting material of the following reactions. Remember, Hydride shifts are possible if/when a more stable carbocation can exist (depending on reaction mechanism)! Put your answers in the indicated boxes d. d. ง HCIarrow_forwardA cylinder contains 12 L of water vapour at 150˚C and 5 atm. The temperature of the water vapour is raised to 175˚C, and the volume of the cylinder is reduced to 8.5 L. What is the final pressure of the gas in atmospheres? assume that the gas is idealarrow_forward
- On the next page is an LC separation of the parabens found in baby wash. Parabens are suspected in a link to breast cancer therefore an accurate way to quantitate them is desired. a. In the chromatogram, estimate k' for ethyl paraben. Clearly indicate what values you used for all the terms in your calculation. b. Is this a "good" value for a capacity factor? Explain. c. What is the resolution between n-Propyl paraben and n-Butyl paraben? Again, indicate clearly what values you used in your calculation. MAU | Methyl paraben 40 20 0 -2 Ethyl paraben n-Propyl paraben n-Butyl paraben App ID 22925 6 8 minarrow_forwardd. In Figure 4, each stationary phase shows some negative correlation between plate count and retention factor. In other words, as k' increases, N decreases. Explain this relationship between k' and N. Plate Count (N) 4000 3500 2500 2000 1500 1000 Figure 4. Column efficiency (N) vs retention factor (k') for 22 nonionizable solutes on FMS (red), PGC (black), and COZ (green). 3000 Eluent compositions (acetonitrile/water, A/W) were adjusted to obtain k' less than 15, which was achieved for most solutes as follows: FMS (30/70 A/W), PGC (60/40), COZ (80/20). Slightly different compositions were used for the most highly retained solutes. All columns were 50 mm × 4.6 mm id and packed with 5 um particles, except for COZ, which was packed with 3 um particles. All other chromatographic conditions were constant: column length 5 cm, column j.§. 4.6 mm, flow rate 2 mL/min, column temperature 40 °C, and injection volume 0.5 μL Log(k'x/K'ethylbenzene) FMS 1.5 1.0 0.5 0.0 ཐྭ ཋ ཤྩ བྷྲ ; 500 0 5 10…arrow_forwardf. Predict how the van Deemter curve in Figure 7 would change if the temperature were raised from 40 °C to 55 °C. Figure 7. van Desmter curves in reduced coordinates for four nitroalkane homologues (nitropropane, black; nitrobutane, red; nitropentane, blue; and nitrohexane, green) separated on the FMS phase. Chromatographic conditions: column dimensions 50 mm × 4.6 mm id, eluent 30/70 ACN/water, flow rates 0.2-5.0 mL/min, injection volume 0.5 and column temperature 40 °C. No corrections to the plate heights have been made to account for extracolumn dispersion. Reduced Plate Height (h) ° 20 40 60 Reduced Velocity (v) 8. (2) A water sample is analyzed for traces of benzene using headspace analysis. The sample and standard are spiked with a fixed amount of toluene as an internal standard. The following data are obtained: Ppb benzene Peak area benzene Peak area toluene 10.0 252 376 Sample 533 368 What is the concentration of benzene in the sample?arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning




