
(a)
Interpretation:
Which
Concept Introduction:
Hydaration reaction is nothing but the addition of water to an alkene. In this reaction two bonds are simultaneously broken, one is carbon-carbon double bond, and second one is
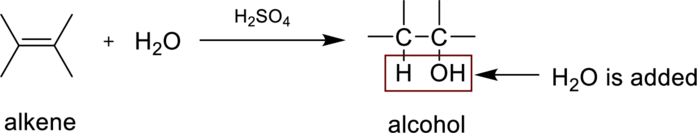
In the addition of water to an unsymmetrical alknene, the hydrogen from water is bonded to the less substituted carbon atom and is called Markovnikov’s rule.
(b)
Interpretation:
Which alkene as a starting material used to prepare cyclohexanol has to be determined.
Concept Introduction:
Hydaration reaction is nothing but the addition of water to an alkene. In this reaction two bonds are simultaneously broken, one is carbon-carbon double bond, and second one is
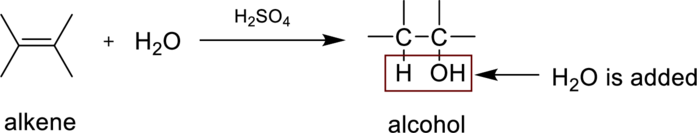
In the addition of water to an unsymmetrical alknene, the hydrogen from water is bonded to the less substituted carbon atom and is called Markovnikov’s rule.
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





