
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
2nd Edition
ISBN: 9780077633707
Author: Janice Smith
Publisher: Mcgraw-hill Higher Education (us)
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 11, Problem 11.27UKC
Answer the following questions about compound A, represented in the given ball-and-stick model.
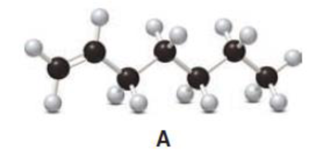
- a. Convert A to a condensed structure and give its IUPAC name.
- b. What product is formed when A is treated with H2 in the presence of a metal catalyst?
- c. What product is formed when A is treated with H2O in the presence of H2SO4?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Give the IUPAC name for each compound.
CH3
CH2CH3
Br
a.
PHCH(CH3)2
b.
С.
d.
Draw the products formed when phenol(C6H5OH) is treated with each reagent. Give an explanation.
d. (CH3CH2)2CHCOCl, AlCl3
j. product in (d), then NH2NH2, –
OH
Draw the products formed when each alkene is treated with Grubbs catalyst
Chapter 11 Solutions
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
Ch. 11.1 - Convert each condensed structure to a complete...Ch. 11.1 - Determine whether each molecular formula...Ch. 11.1 - Give the molecular formula for each of the...Ch. 11.2 - Give the IUPAC name for each alkene. a. (CH3CH2)2C...Ch. 11.2 - Prob. 11.5PCh. 11.2 - Give the structure corresponding to each name. a....Ch. 11.3 - Prob. 11.7PCh. 11.3 - Bombykol is secreted by the female silkworm moth...Ch. 11.3 - Prob. 11.9PCh. 11.3 - Prob. 11.10P
Ch. 11.3 - Prob. 11.11PCh. 11.5 - Prob. 11.12PCh. 11.5 - Prob. 11.13PCh. 11.6 - Prob. 11.14PCh. 11.6 - Prob. 11.15PCh. 11.7 - Prob. 11.16PCh. 11.7 - Prob. 11.17PCh. 11.9 - Prob. 11.18PCh. 11.9 - Draw the structure corresponding to each name. a....Ch. 11.10 - Prob. 11.20PCh. 11.10 - Prob. 11.21PCh. 11.10 - Prob. 11.22PCh. 11 - Prob. 11.23UKCCh. 11 - Prob. 11.24UKCCh. 11 - Prob. 11.25UKCCh. 11 - Prob. 11.26UKCCh. 11 - Answer the following questions about compound A,...Ch. 11 - Prob. 11.28UKCCh. 11 - Prob. 11.29UKCCh. 11 - Prob. 11.30UKCCh. 11 - Prob. 11.31UKCCh. 11 - Prob. 11.32UKCCh. 11 - Prob. 11.33APCh. 11 - Prob. 11.34APCh. 11 - Prob. 11.35APCh. 11 - Prob. 11.36APCh. 11 - Prob. 11.37APCh. 11 - Falcarinol is a natural pesticide found in carrots...Ch. 11 - Prob. 11.39APCh. 11 - Prob. 11.40APCh. 11 - Prob. 11.41APCh. 11 - Prob. 11.42APCh. 11 - Prob. 11.43APCh. 11 - Give the structure corresponding to each IUPAC...Ch. 11 - Leukotriene C4 is a key compound that causes the...Ch. 11 - Prob. 11.46APCh. 11 - Prob. 11.47APCh. 11 - Prob. 11.48APCh. 11 - Prob. 11.49APCh. 11 - Prob. 11.50APCh. 11 - Prob. 11.51APCh. 11 - Prob. 11.52APCh. 11 - Prob. 11.53APCh. 11 - Prob. 11.54APCh. 11 - Prob. 11.55APCh. 11 - Prob. 11.56APCh. 11 - Prob. 11.57APCh. 11 - Draw the products formed in each reaction.Ch. 11 - Prob. 11.59APCh. 11 - Prob. 11.60APCh. 11 - Prob. 11.61APCh. 11 - Prob. 11.62APCh. 11 - Prob. 11.63APCh. 11 - Prob. 11.64APCh. 11 - Prob. 11.65APCh. 11 - Prob. 11.66APCh. 11 - Prob. 11.67APCh. 11 - Prob. 11.68APCh. 11 - Prob. 11.69APCh. 11 - Prob. 11.70APCh. 11 - Prob. 11.71APCh. 11 - Prob. 11.72APCh. 11 - Prob. 11.73APCh. 11 - Prob. 11.74APCh. 11 - Prob. 11.75APCh. 11 - Prob. 11.76APCh. 11 - Prob. 11.77APCh. 11 - Prob. 11.78APCh. 11 - Prob. 11.79APCh. 11 - Prob. 11.80APCh. 11 - Prob. 11.81APCh. 11 - Prob. 11.82APCh. 11 - Prob. 11.83APCh. 11 - Prob. 11.84APCh. 11 - Prob. 11.85APCh. 11 - Prob. 11.86APCh. 11 - Are cis-2-hexene and trans-3-hexene constitutional...Ch. 11 - Prob. 11.88CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the products formed when each compound is treated with HNO3 and H2SO4.arrow_forwardAnswer each question using the ball-and-stick model of compound A. Draw a stereoisomer for A and give its IUPAC name.Draw a constitutional isomer that contains an OH group and give itsIUPAC name.arrow_forwardWhat products are formed when benzene is treated with each alkyl chloride and AlCl3?arrow_forward
- Which alcohols can be prepared as a single product by hydroboration– oxidation of an alkene? Which alcohols can be prepared as a single product by the acid-catalyzed addition of H2O to an alkene?arrow_forwardDraw the eight constitutional isomers having the molecular formula C5H11Cl.a. Give the IUPAC name for each compound (ignoring R and S designations).b. Classify each alkyl halide as 1°, 2°, or 3°.c. Label any stereogenic centers.d. For each constitutional isomer that contains a stereogenic center, draw all possible stereoisomers, and label each stereogenic center as R or S.arrow_forwardDraw the eight constitutional isomers having the molecular formula C5H11Cl. a.Give the IUPAC name for each compound (ignoring R and S designations). b.Classify each alkyl halide as 1°, 2°, or 3°. c.Label any stereogenic centers. d.For each constitutional isomer that contains a stereogenic center, draw all possible stereoisomers, and label each stereogenic center as R or S.arrow_forward
- Draw the products (including stereoisomers) formed when benzaldehyde (C6H5CHO) is treated with each Wittig reagent.arrow_forward• Whał arc the IUPAC namar of the ff. Carboxylic acias? a. COOH COOH CH3 b. I f. .COOH CHJCH2 ÇHCHCOOH CH3 NO2 tON C. COOH ноос d. COOHarrow_forwardHow is iupac name? ww.h w c) H-C-OHarrow_forward
- Draw the products formed when each alkyl halide is treated with NaCN.arrow_forwardProvide the two isomers of compound A. Please provide the IUPAC name for each. 1st isomer: has quarternary, secondary, and primary carbons 2nd isomer: has secondary and primary carbonsarrow_forwardDraw the structure of A? NaNH2 H i Liq. NH3 Aarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License