
Interpretation: The experiment which concludes that the atom contains a heavy positively charged nucleus which is surrounded by electrons is to be described.
Concept introduction: Rutherford’s atomic model describes that the nucleus of an atom is small and dense and possess positive charge and the electrons with negative charge revolves around the nucleus in a circular motion.
Answer to Problem 1A
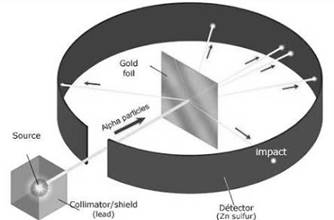
Rutherford’s gold foil experiment.
Explanation of Solution
Rutherford took a radioactive element that decays naturally and emits alpha particles and enclosed it in a container with a hole in it through which a beam of alpha particles comes out. He placed a gold foil in which the beam strikes.
He noticed that most of the beam passes through while some bents at large angles and some bounced back. This can be possible only when the atom has a small and heavy region of positive charge in it.
There were a lot of theories which Rutherford failed to explain in his gold foil experiment.
Want to see more full solutions like this?
Chapter 11 Solutions
World of Chemistry, 3rd edition
- QUESTION: Fill in the answers in the empty green boxes 1. Step 2 2. Step 3 3. Step 4 (SUM) 4. Step 5 (df) (GIVEN) 5. Determine S y/x value *The data values have been provided in the worksheet attached in the first image*arrow_forwardIf the symbol A is placed in a reaction, at what temperature does it take place?arrow_forwardBy malonic or acetylacetic synthesis, synthesize 3-methyl-4-oxopentanoic acid (indicate the formulas of the compounds).arrow_forward
- oalmitic acid is a 16 carbon acid. In a balanced equation, the products of the sponification of tripalmitin (glyceryl tripalmitate are blank.arrow_forwardWrite the esterification reaction mechanism of salicylic acid and acetic acid to produce aspirin (acetylsalicylic acid). Note: salicylic acid will act as the alcoholarrow_forwardWhat type of interaction would you expect between the following R groups in the tertiary structure of a protein? O -CH2-CO and -CH2-CH2-CH2-CH2-NH3+ a. disulfide bonds b. salt bridges c. hydrogen bonds HO abios vist anisinoo tedt bigil s ai loistaslor sale! 10 OUT d. hydrophobic interactions e. peptide bondsarrow_forward
- 4. True or false: This skeletal structure represents a saturated fatty acid. Ini to 0 fale) me OH faistong starrow_forwardBy malonic or acetylacetic synthesis, synthesize 5-Methyl-2-hexanone (with the formulas of the compounds).arrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' by filling in all the empty green boxes *The values are all provided in the first photo attached*arrow_forward
- Draw the formula for 3-chlorobenzoic acetic anhydride.arrow_forwardBy malonic or acetylacetic synthesis, synthesize 2-methylbutanoic acid (indicate the formulas of the compounds).arrow_forwardObtain 2-methylbutanoic acid by malonic or acetylacetic synthesis (indicate the formulas of the compounds involved).arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





