
Suppliers of radioisotopically labeled compounds usually provide each product as a mixture Of labeled and unlabeled material. Unlabeled material is added deliberately as a carrier, partly because the specific activity of the carrier-free product is too high to be useful and partly because the product is more stable at lower specific activities. Using the radioactive decay law, calculate the
following.
a. The specific activity Of carrier-free [22P]-orthophosphate, in mCi/mmol.
b. The fraction Of H atoms that are radioactive in a preparation Of uniform-label [3H]-leucine, provided at 10 mCi/mmol.
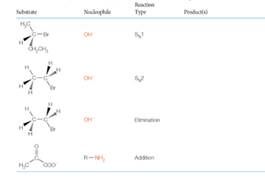
Predict the product(s) of the following reactions:
Learn your wayIncludes step-by-step video

Chapter 11 Solutions
Biochemistry: Concepts and Connections (2nd Edition)
Additional Science Textbook Solutions
Anatomy & Physiology (6th Edition)
Microbiology: An Introduction
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
Human Biology: Concepts and Current Issues (8th Edition)
Chemistry: A Molecular Approach (4th Edition)
Campbell Essential Biology (7th Edition)
- 1. (10 points) Pulverized coal pellets, which may be ° approximated as carbon spheres of radius r = 1 mm, are burned in a pure oxygen atmosphere at 1450 K and 1 atm. Oxygen is transferred to the particle surface by diffusion, where it is consumed in the reaction C + O₂ →> CO₂. The reaction rate is first order and of the form No2 = k₁C₁₂(r), where k₁ = 0.1 m/s. Neglecting changes in r, determine the steady-state O₂ molar consumption rate in kmol/s. At 1450 K, the binary diffusion coefficient for O2 and CO2 is 1.71 x 10ª m²/s.arrow_forward2. (20 points) Consider combustion of hydrogen gas in a mixture of hydrogen and oxygen adjacent to the metal wall of a combustion chamber. Combustion occurs at constant temperature and pressure according to the chemical reaction 2H₂+ O₂→ 2H₂O. Measurements under steady-state conditions at 10 mm from the wall indicate that the molar concentrations of hydrogen, oxygen, and water vapor are 0.10, 0.10, and 0.20 kmol/m³, respectively. The generation rate of water vapor is 0.96x102 kmol/m³s throughout the region of interest. The binary diffusion coefficient for each of the species (H, O̟, and H₂O) in the remaining species is 0.6 X 10-5 m²/s. (a) Determine an expression for and make a qualitative plot of C as a function of distance from the wall. H2 (b) Determine the value of C2 at the wall. H2 (c) On the same coordinates used in part (a), sketch curves for the concentrations of oxygen and water vapor. This will require you to calculate Co, and C. 02 H20 (d) What is the molar flux of water…arrow_forward4. (15 points) Consider a spherical organism of radius ro within which respiration occurs at a uniform volumetric rate of That is, oxygen (species A) consumption is governed by a first- order reaction, homogeneous chemical reaction. a. If a molar concentration of CA(ro) = CA,o is maintained at the surface of the organism, obtain an expression for the radial distribution of oxygen, CA(r), within the organism. Hint: To simplify solution of the species diffusion equation, invoke the transformation y = rCA. b. Obtain an expression for the rate of oxygen consumption within the organism. c. Consider an organism of radius ro = 0.10 mm and a diffusion coefficient of DAB = 108 m2/s. If CA, o = 5 x105 kmol/m3 and k1 20 s1, estimate the corresponding value of the molar concentration at the center of the organism. What is the rate of oxygen consumption by the organism?arrow_forward
- 3. (15 points) Living cells homogeneously distributed (immobilized) with an agarose gel require glucose to survive. An important aspect of the biochemical system design is the effective diffusion coefficient of glucose (A) into the cell- immobilized gel. Consider the experiment shows below where a slab of the cell-immobilized gel of 1.0cm thickness is placed within a well-mixed aqueous solution of glucose maintained at a concentration of 50 mmol/L. The glucose consumption within the cell-immobilized gel proceeds by a zero-order process given by R₁ = -0.05 mmol/(L min). The solubilities of glucose in both the water and the gel are the same; that is, the concentration of the glucose on the water side of the water-gel interface is equal to the concentration of the glucose on the gel side of the water gel interface. A syringe is mounted at the center of the gel carefully excises a tiny sample of the gel for glucose analysis. A Well mixed solution Constant concentration 50nmol/L Living…arrow_forwardTwo tetrapeptides were isolated from a possum's sweat glands. These peptides were sequenced using Edman degradation and the following 2 sequences were obtained: Gly-Asp-Ala-Leu Gly-Asp-Asp-Leu Can you please help show the titration curve for both of these peptides and calculate the PI?arrow_forwardTwo tetrapeptides were isolated from a possum's sweat glands. These peptides were sequenced using Edman degradation and the following 2 sequences were obtained: Gly-Asp-Ala-Leu Gly-Asp-Asp-Leu What is the structure of the PTH derivative produced during the last round of amino acid sequencing?arrow_forward
- What is the primary sequence of this undecapeptide? Also, if x-ray crystallography shows a highly stable hairpin turn within the polypeptide, what about the primary sequence explains this structural feature?arrow_forwardDraw the product of this reaction. Ignore inorganic byproducts. H H ⚫OH HO- -H H- -OH H- -OH CH2OH Ag*, NH4OH, H2O Draw Fischer Projectionarrow_forwardDraw the product of this reaction. Ignore inorganic byproducts. H₂O -OH H ⚫OH HO H HO- CH2OH Cu2+ Draw Fischer Projectionarrow_forward
- Draw the product of this reaction. Ignore inorganic byproducts. H、 H -OH H ⚫OH H -OH CH2OH Fehlings' solution ⑤ Draw Fischer Projectionarrow_forwardDraw the product of this reaction. Ignore inorganic byproducts. HO C=0 H ⚫OH H ⚫OH HO- H HO H CH2OH Tollens' solution Draw Fischer Projectionarrow_forwardDraw the product of this reaction. Ignore inorganic byproducts. H-C=O HO H HO H H- ⚫OH HO H CH2OH HNO3, H2O Draw Fischer Projectionarrow_forward
- Essentials of Pharmacology for Health ProfessionsNursingISBN:9781305441620Author:WOODROWPublisher:Cengage
 Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning





