
Concept explainers
Answer the following questions about aldosterone, a compound that helps to control the absorption of Na+and Cl- ions in the kidneys and, as a result, affects water retention.
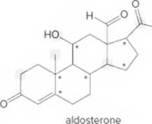
- Identify the
functional groups. - Draw in all lone pairs on O atoms.
- How many C’s does aldosterone contain?
- How many H’s are present at each C labeled with an asterisk (*)?
- Give the shape around each atom labeled in gray.
- Label all polar bonds.
(a)
Interpretation:
The functional group in aldosterone should be midentified.
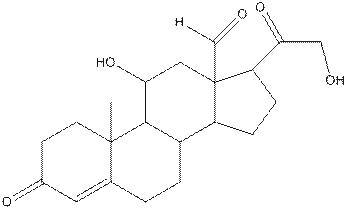
Concept Introduction:
Functional groups are the atoms or group of atoms that gives chemical properties to an organic compound and also chemical reactivity centre. Examples of functional groups are aldehyde, ketone, alcohol, double bond.
Answer to Problem 11.87P
In aldosterone, functional groups present are alcohol, aldehyde, ketone and alkene.
Explanation of Solution
Functional groups are the atoms or group of atoms that gives chemical properties to an organic compound and also chemical reactivity centre. Structures of different functional groups are as follows:
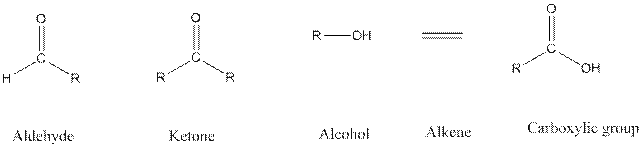
R represents any alkyl chain.
In functional group ketone carbonyl carbon is bonded to two alkyl groups, in aldehyde carbonyl carbon is attached to one alkyl group and one hydrogen atom, in alcohol functional group an −OH is bonded to an alkyl group.
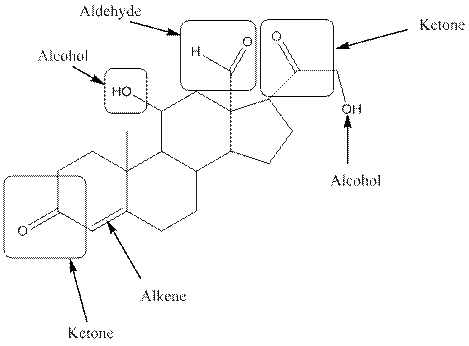
The compound aldosterone consists of two alcohol frunctional group, two ketone functional groups and one aldehyde functional group and one alkene. So, four types of functional group, that is, alcohol, aldehyde, ketone and alkene.
(b)
Interpretation:
All the lone pairs on oxygen atom of aldosterone should be drawn.
Concept Introduction:
Oxygen atom has six valence electrons. The electrons which are used in bond formation are called bonding electrons. The electrons that remain on an atom after bond formation are called lone pair electrons.
Answer to Problem 11.87P
The structure of aldosterone having all lone pairs on oxygen atom is,
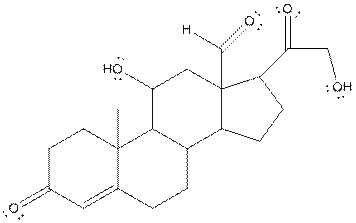
Explanation of Solution
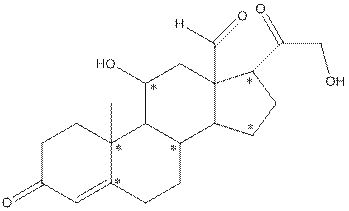
The structure of aldosterone is as follows:
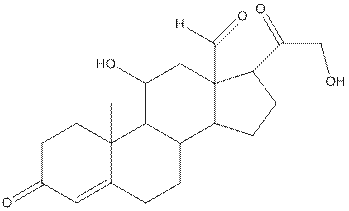
In the compound, three double bonded oxygen atoms present. Oxygen has six valence electrons. Two electrons in all double bonded oxygen atoms are used for formation of double bond. Remaining four electrons present as two lone pair. Also the compound contains three single bonded oxygen atoms. One electron is used for making O-H bond and one electron is used for making O-C bond. Remaining four electrons present as two lone pairs on each single bonded oxygen atom. Hence, the structure having all lone pairs on oxygen atom is,
(c)
Interpretation:
The number of carbon atoms in the structure of aldosterone should be determined.
Concept Introduction:
In skeletal structure the terminals represent methyl
Answer to Problem 11.87P
Twenty one carbon atoms present in the aldosterone.
Explanation of Solution
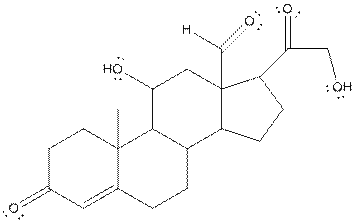
The structure of aldosterone is given as follows:
In skeletal structure the terminals represent methyl
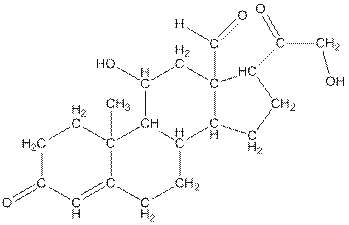
Hence, twenty one carbon atoms present in the compound.
(d)
Interpretation:
The number of hydrogen atoms present in the * carbon atoms in aldosterone should be determined.
Concept Introduction:
In skeletal structure the terminals represent methyl
Answer to Problem 11.87P
The * carbon atoms have total five hydrogen atoms.
Explanation of Solution
In skeletal structure the terminals represent methyl
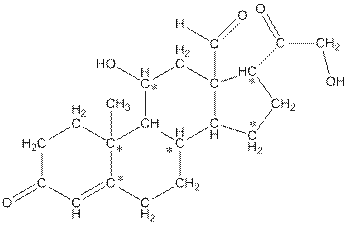
Hence, the * carbon atoms have total five hydrogen atoms.
(e)
Interpretation:
The shape around each indicated carbon atom in aldosterone should be determined.
Concept Introduction:
The following table should be used while determining the shape around an atom.
| Number of groups | Number of atoms | Number of lone pairs | Shape | Bond angle |
| 2 | 2 | 0 | Linear | |
| 3 | 3 | 0 | Trigonal planar | |
| 4 | 4 | 0 | Tetrahedral | |
| 4 | 3 | 1 | Trigonal pyramidal | |
| 4 | 2 | 2 | Bent |
Answer to Problem 11.87P
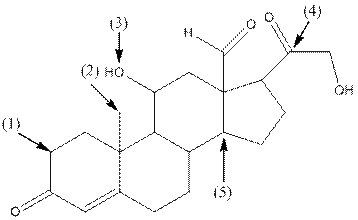
- Shape around carbon (1) is tetrahedral, shape around carbon (2) is tetrahedral, shape around oxygen (3) is bent, shape around carbon (4) is trigonal planar and shape around carbon (5) is tetrahedral.
Explanation of Solution
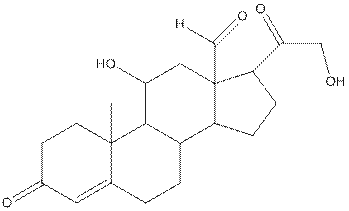
The complete structure of aldosterone is as follows:
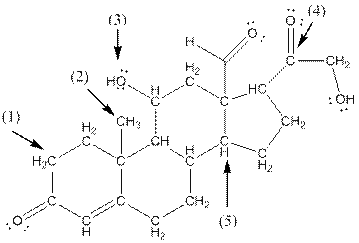
- Carbon (1) has four groups ( two hydrogen and two carbon) surrounding it. So, shape around carbon (1) is tetrahedral.
- Carbon (2) has four groups (three hydrogen and one carbon) surrounding it. So, shape around carbon (2) is tetrahedral.
- Oxygen (3) has two groups (one carbon and one hydrogen) and two lone pairs surrounding it. So, shape around oxygen (3) is bent.
- Carbon (4) has three groups (two carbon and one oxygen) surrounding it. So, shape around carbon (4) is trigonal planar.
- Carbon (5) has four groups (three carbon and one hydrogen) surrounding it. So, shape around carbon (5) is tetrahedral.
(f)
Interpretation:
All the polar bonds in aldosterone should be labeled.
Concept Introduction:
The unequal sharing of valence electrons in a bond is called polar bond. Polar bond result when the bond formed between two atoms in which one atom is more electronegative than the other one. One example of polar bond is
Structure of HCl is as follows:

In
Answer to Problem 11.87P
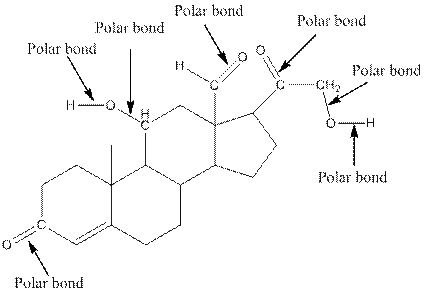
The structure of aldosterone with all polar bonds is as follows:
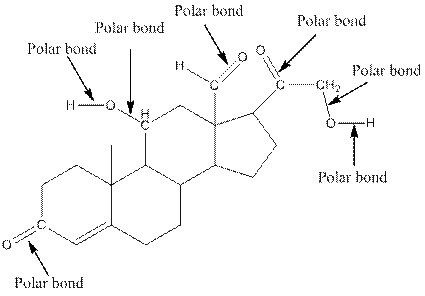
Explanation of Solution
In organic compound, most of the polar bonds formed between carbon and heteroatoms like oxygen, nitrogen, sulphur etc. To identify the polar bonds in the compound aldosterone, find the bonds between carbon and oxygen or oxygen and hydrogen because oxygen is more electronegative than carbon and oxygen is more electronegative than carbon. In aldostrone, five polar bonds are formed between carbon and oxygen (oxygen is more electronegative than carbon) and two polar bonds present between oxygen and hydrogen (oxygen is more electronegative than hydrogen). Thus, the polar bonds are:
Want to see more full solutions like this?
Chapter 11 Solutions
General, Organic, & Biological Chemistry
- answer thisarrow_forwardplease add appropriate arrows and tell me in detail where to add which or draw itarrow_forwardPart 1. Draw monomer units of the following products and draw their reaction mechanism (with arrow pushing) Temporary cross-linked polymer Using: 4% polyvinyl alcohol+ methyl red + 4% sodium boratearrow_forward
- can you please answer both these questions and draw the neccesaryarrow_forwardcan you please give the answer for both these pictures. thankyouarrow_forwardPart 1. Draw monomer units of the following products and draw their reaction mechanism (with arrow pushing) | Bakelite like polymer Using: Resorcinol + NaOH + Formalinarrow_forward
- Question 19 0/2 pts 3 Details You have a mixture of sodium chloride (NaCl) and potassium chloride (KCl) dissolved in water and want to separate out the Cl- ions by precipitating them out using silver ions (Ag+). The chemical equation for the net ionic reaction of NaCl and KCl with silver nitrate, AgNO3, is shown below. Ag+(aq) + Cl(aq) → AgCl(s) The total mass of the NaCl/KCl mixture is 1.299 g. Adding 50.42 mL of 0.381 M solution precipitates out all of the Cl-. What are the masses of NaCl and KCl in the mixture? Atomic masses: g: Mass of NaCl g: Mass of KCL Ag = 107.868 g mol- 1 Cl = 35.453 g mol- 1 K = 39.098 g mol- N = 14.007 g mol−1 Na = 22.99 g mol−1 0 = 15.999 g mol 1 Question Help: ✓ Message instructor Submit Questionarrow_forwardPart 1. Draw monomer units of the following products and draw their reaction mechanism (with arrow pushing) Polyester fiber Using a) pthalic anhydride + anhydrous sodium acetate + ethylene glycol B)pthalic anhydride + anhydrous sodium acetate + glycerolarrow_forwardIdentify the missing starting materials/ reagents/ products in the following reactions. Show the stereochemistry clearly in the structures, if any. If there is a major product, draw the structures of the major product with stereochemistry clearly indicated where applicable. Show only the diastereomers (you do not have to draw the pairs of enantiomers). If you believe that multiple products are formed in approximately equal amounts (hence neither is the major product), draw the structures of the products, and show the detailed mechanism of these reactions to justify the formation of the multiple products. If you believe no product is formed, explain why briefly. (6 mark for each, except f and g, which are 10 mark each)arrow_forward
- 3. What starting material would you use to synthesize 3-hydroxypentanoic acid using a NaBH4 reduction?arrow_forward1. Give stereochemical (Fischer projection) formulas for all (but no extras) the stereoisomers that could theoretically form during the reduction of a. the carbonyl group of 2-methyl-3--pentanone b. both carbonyl groups of 2,4-pentanedione (careful!) 2. Predict the products of the reduction of O=CCH2CH2CH2C=O with a. LiAlH4 b. NaBH4 CH3 OHarrow_forwardWhich of the following compounds can be synthesized using one reaction from any alkene, as a major product? If it can be synthesized, propose a route, and you may use any other starting materials, reagents and solvents as needed. If you do not think that it can be synthesized as a major product from an alkene, explain in detail why.arrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

