
Concept explainers
Benzocaine is the active ingredient in topical pain relievers such as Orajel and Anbesol. Give the molecular formula for benzocaine.
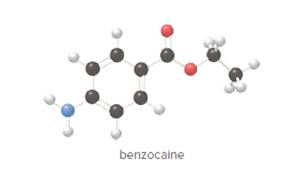
- Give the molecular formula for benzocaine.
- Draw in all lone pairs on heteroatoms using a skeletal structure.
- How many trigonal planar carbons does benzocaine contain?
- Predict the water solubility of benzocaine.
- Label all polar bonds.
(a)
Interpretation:
To determine the molecular formula of benzocaine from the ball and stick model.
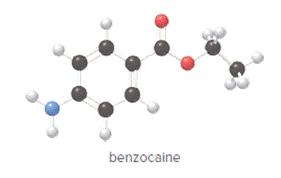
Concept Introduction:
In ball and stick model, black color ball represents carbon atom, white color ball represents hydrogen atom, red color ball represents oxygen atom and blue color ball represents nitrogen atom. To determine the molecular formula, convert the ball and stick model to complete structure.
Answer to Problem 11.85P
Molecular formula of benzocaine is
Explanation of Solution
The compound is as follows:
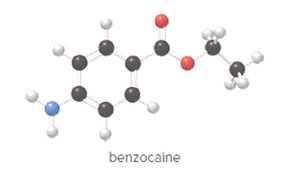
Convert ball and stick model to normal structure. Replace black balls by carbon atoms, red balls by oxygen atoms, blue ball by nitrogen atom and whit balls by hydrogen atom. But, all the bonds between atoms are same.
In benzocaine, nine carbon atoms, eleven hydrogen atoms, two oxygen atoms and one nitrogen atom present. Hence, molecular formula of benzocaine is
(b)
Interpretation:
To draw all lone pairs on heteroatoms of benzocaine using a skeletal structure.
Concept Introduction:
Heteroatoms are those atoms in an organic compound other than carbon and hydrogen atom like oxygen, nitrogen, etc. Lone pairs are pair of electrons available on an atom after bond formation.
Answer to Problem 11.85P
The structure of benzocaine having all lone pairs on heteroatoms is as follows:
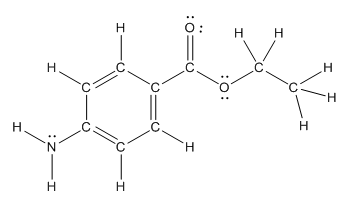
Explanation of Solution
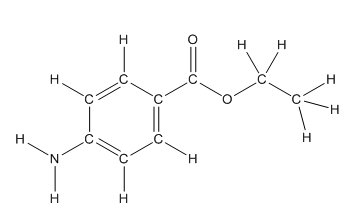
Skeletal structure of benzocaine is as follows:
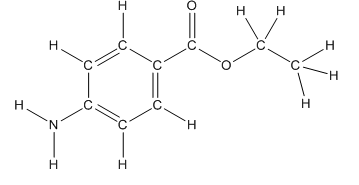
The heteroatoms present in benzocaine are nitrogen atom and two oxygen atoms. Nitrogen has five valence electrons. Out of five electrons, two electrons are used for making two (N-H) bonds, one electron is used for making one N-C bond. Remaining two electrons forms a lone pair. Oxygen has six valence electrons. For double bonded oxygen atom, two electrons are used for making the double bond. Remaining four electrons forms two lone pair. Other oxygen atom forms two bonds with carbon atoms means two electrons are used in bond formation. So, two pairs of lone pair present. Hence, the structure having all lone pairs on heteroatoms is as follows:
(c)
Interpretation:
To determine the number of carbon atoms having trigonal planar shape in benzocaine.
Concept Introduction:
The following table should be used while determining shape around an atom.
| Number of groups | Number of atoms | Number of lone pairs | Shape | Bond angle |
| 2 | 2 | 0 | Linear | |
| 3 | 3 | 0 | Trigonal planar | |
| 4 | 4 | 0 | Tetrahedral | |
| 4 | 3 | 1 | Trigonal pyramidal | |
| 4 | 2 | 2 | Bent |
If an atom is surrounded by three groups, then the shape around that particular atom is trigonal planar.
Answer to Problem 11.85P
In benzocaine, seven carbon atoms have trigonal planar structure.
Explanation of Solution
If an atom is surrounded by three groups, then the shape around that particular atom is trigonal planar.
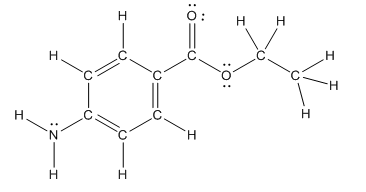
Structure of benzocaine is as follows:
To find the number of carbon atoms having trigonal planar shape in benzocaine, observe the carbon atoms and find which carbon atom has three groups surround it.
All the carbon atoms in the benzene ring have three atoms around them also the carbon bonded to the benzene ring has three groups surround it. So, seven carbon atoms in benzocaine have trigonal planar structure.
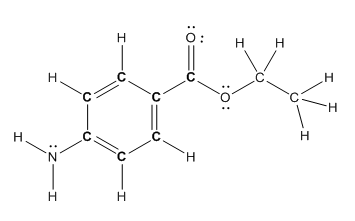
The bold carbon atoms have trigonal planar structure.
(d)
Interpretation:
To predict the solubility of benzocaine in water.
Concept Introduction:
Water is a polar solvent. To dissolve in water the compound must be polar. Polar compound is that compound in which polar bonds are present. The unequal sharing of valence electrons in a bond is called polar bond. Polar bond result when the bond formed between two atoms in which one atom is more electronegative than the other one. One example of polar bond is
Structure of HCl is as follows:

In
Answer to Problem 11.85P
Benzocaine is water soluble compound.
Explanation of Solution
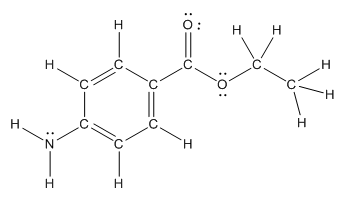
The structure of benzocaine is as follows:
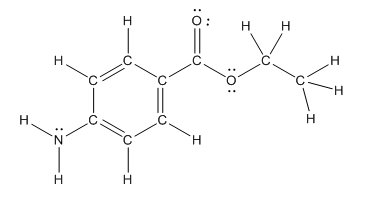
As the compound contains heteroatoms like nitrogen, oxygen the compound is polar that is, they can form hydrogen bonds with water. So, benzocaine is water soluble compound.
(e)
Interpretation:
To label all the polar bonds in benzocaine.
Concept Introduction:
The unequal sharing of valence electrons in a bond is called polar bond. Polar bond result when the bond formed between two atoms in which one atom is more electronegative than the other one. One example of polar bond is
Structure of HCl is as follows:

In
Answer to Problem 11.85P
Polar bond in benzocaine are as follows:
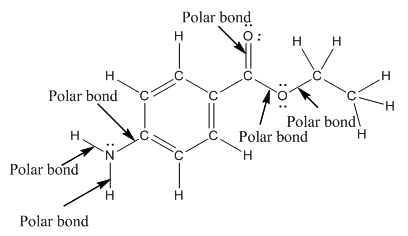
Explanation of Solution
The structure of benzocaine is as follows:
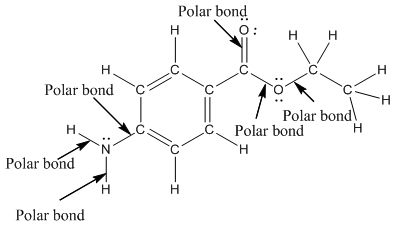
In organic compound, most of the polar bonds formed between carbon and heteroatoms like oxygen, nitrogen, sulphur etc. In benzocaine six polar bonds present that is, three polar bond between carbon and oxygen (oxygen is more electronegative than carbon), two polar bond between nitrogen and hydrogen (nitrogen is more electronegative than hydrogen) and on polar bond between carbon and nitrogen (nitrogen is more electronegative than carbon).
Want to see more full solutions like this?
Chapter 11 Solutions
General, Organic, & Biological Chemistry
- What is the IUPAC name of the following compound? CH₂CH₂ H CI H₂CH₂C H CH₂ Selected Answer: O (35,4R)-4 chloro-3-ethylpentane Correctarrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electrons-pushing arrows for the following reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. I I I H Select to Add Arrows HCI, CH3CH2OHarrow_forward
- Curved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and the follow the arrows to draw the intermediate and product in this reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the curved arrows to draw the intermediates and product of the following reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the arrows to draw the intermediate and the product in this reaction or mechanistic step(s).arrow_forward
- Look at the following pairs of structures carefully to identify them as representing a) completely different compounds, b) compounds that are structural isomers of each other, c) compounds that are geometric isomers of each other, d) conformers of the same compound (part of structure rotated around a single bond) or e) the same structure.arrow_forwardGiven 10.0 g of NaOH, what volume of a 0.100 M solution of H2SO4 would be required to exactly react all the NaOH?arrow_forward3.50 g of Li are combined with 3.50 g of N2. What is the maximum mass of Li3N that can be produced? 6 Li + N2 ---> 2 Li3Narrow_forward
- 3.50 g of Li are combined with 3.50 g of N2. What is the maximum mass of Li3N that can be produced? 6 Li + N2 ---> 2 Li3Narrow_forwardConcentration Trial1 Concentration of iodide solution (mA) 255.8 Concentration of thiosulfate solution (mM) 47.0 Concentration of hydrogen peroxide solution (mM) 110.1 Temperature of iodide solution ('C) 25.0 Volume of iodide solution (1) used (mL) 10.0 Volume of thiosulfate solution (5:03) used (mL) Volume of DI water used (mL) Volume of hydrogen peroxide solution (H₂O₂) used (mL) 1.0 2.5 7.5 Time (s) 16.9 Dark blue Observations Initial concentration of iodide in reaction (mA) Initial concentration of thiosulfate in reaction (mA) Initial concentration of hydrogen peroxide in reaction (mA) Initial Rate (mA's)arrow_forwardDraw the condensed or line-angle structure for an alkene with the formula C5H10. Note: Avoid selecting cis-/trans- isomers in this exercise. Draw two additional condensed or line-angle structures for alkenes with the formula C5H10. Record the name of the isomers in Data Table 1. Repeat steps for 2 cyclic isomers of C5H10arrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





