
Concept explainers
(a)
Interpretation:
To identify the incorrect part present in 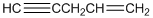 structure.
structure.
Concept Introduction:
The skeletal formula of a compound is also known as shorthand molecular formula. It gives details of its molecules bonding and geometry of molecule.
(b)
Interpretation:
To identify the incorrect part present in 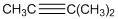 structure.
structure.
Concept Introduction:
The skeletal formula of a compound is also known as shorthand molecular formula. It gives details of its molecules bonding and geometry of molecule.
(c)
Interpretation:
To identify the incorrect part present in 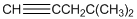 structure.
structure.
Concept Introduction:
The skeletal formula of a compound is also known as shorthand molecular formula. It gives details of its molecules bonding and geometry of molecule.
(d)
Interpretation:
To identify the incorrect part present in the following structure:
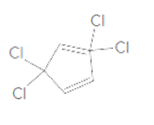
Concept Introduction:
The skeletal formula of a compound is also known as shorthand molecular formula. It gives details of its molecules bonding and geometry of molecule.
(e)
Interpretation:
To identify the incorrect part present in following structure:
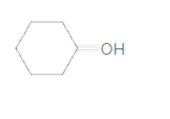
Concept Introduction:
The skeletal formula of a compound is also known as shorthand molecular formula. It gives details of its molecules bonding and geometry of molecule.
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
General, Organic, & Biological Chemistry
- SYNTHESIS REACTIONS. For the following reactions, synthesize the given products from the given reactants. Multiple reactions/steps will be needed. For the one of the steps (ie reactions) in each synthesis, write out the mechanism for that reaction and draw an energy diagram showing the correct number of hills and valleys for that step's mechanism. CI b. a. Use acetylene (ethyne) and any alkyl halide as your starting materials Br C. d. "OH OH III. OHarrow_forwardCalculate the pH and the pOH of each of the following solutions at 25 °C for which the substances ionize completely: (a) 0.200 M HClarrow_forwardCalculate the pH and the pOH of each of the following solutions at 25 °C for which the substances ionize completely: (a) 0.000259 M HClO4arrow_forward
- What is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forwardDetermine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. NaN₃arrow_forwardCan I please get help with this?arrow_forward
- Can I please get help with this?arrow_forwardUse the Henderson-Hasselbalch equation to calculate pH of a buffer containing 0.050M benzoic acidand 0.150M sodium benzoate. The Ka of benzoic acid is 6.5 x 10-5arrow_forwardA. Draw the structure of each of the following alcohols. Then draw and name the product you would expect to produce by the oxidation of each. a. 4-Methyl-2-heptanol b. 3,4-Dimethyl-1-pentanol c. 4-Ethyl-2-heptanol d. 5,7-Dichloro-3-heptanolarrow_forward
- What is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forwardCan I please get help with this.arrow_forwardDetermine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. N₂H₅ClO₄arrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning




