
(a)
Interpretation:
The balanced equation to represent the complete combustion of the given compound is to be stated.
Concept introduction:
Alkane is made of only carbon and hydrogen atoms. These atoms form a bond with each other and with other atoms through the sharing of electrons. They do not completely donate their electrons to other atoms while forming a bond. A general complete combustion reaction of
Answer to Problem 11.60E
The balanced equation to represent the complete combustion of the given compound is shown below as,
Explanation of Solution
The given molecule is butane. The chemical formula of butane is
A general complete combustion reaction of alkanes is shown below as,
Where,
•
The number of carbon atoms in butane is four.
Therefore, the balanced equation to represent the complete combustion of the given compound is shown below as,
The reaction is further simplified as,
The balanced equation to represent the complete combustion of the given compound is shown below as,
(b)
Interpretation:
The balanced equation to represent the complete combustion of the given compound is to be stated.
Concept introduction:
Alkane is made of only carbon and hydrogen atoms. These atoms form a bond with each other and with other atoms through the sharing of electrons. They do not completely donate their electrons to other atoms while forming a bond. A general complete combustion reaction of alkanes is shown below as,
Answer to Problem 11.60E
The balanced equation to represent the complete combustion of the given compound is shown below as,
Explanation of Solution
The given molecule is shown below as,
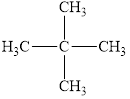
Figure 1
The chemical formula of the given compound is
A general complete combustion reaction of alkanes is shown below as,
Where,
•
The number of carbon atoms in the given compound is five.
Therefore, the balanced equation to represent the complete combustion of the given compound is shown below as,
The balanced equation to represent the complete combustion of the given compound is shown below as,
(c)
Interpretation:
The balanced equation to represent the complete combustion of the given compound is to be stated.
Concept introduction:
Alkane is made of only carbon and hydrogen atoms. These atoms form a bond with each other and with other atoms through the sharing of electrons. They do not completely donate their electrons to other atoms while forming a bond. A general complete combustion reaction of cycloalkanes is shown below as,
Answer to Problem 11.60E
The balanced equation to represent the complete combustion of the given compound is shown below as,
Explanation of Solution
The given molecule is shown below as,
![]()
Figure 2
The chemical formula of the given compound is
A general complete combustion reaction of cycloalkanes is shown below as,
Where,
•
The number of carbon atoms in the given compound is four.
Therefore, the balanced equation to represent the complete combustion of the given compound is shown below as,
The balanced equation to represent the complete combustion of the given compound is shown below as,
Want to see more full solutions like this?
Chapter 11 Solutions
Chemistry for Today: General, Organic, and Biochemistry
- In three dimensions, explain the concept of the velocity distribution function of particles within the kinetic theory of gases.arrow_forwardIn the kinetic theory of gases, explain the concept of the velocity distribution function of particles in space.arrow_forwardIn the kinetic theory of gases, explain the concept of the velocity distribution function of particles.arrow_forward
- Hi!! Please provide a solution that is handwritten. this is an inorganic chemistry question please answer accordindly!! its just one question with parts JUST ONE QUESTION with its parts spread out till part (g), please answer EACH part till the end and dont just provide wordy explanations wherever asked for structures, please DRAW DRAW them on a paper and post clearly!! answer the full question with all calculations step by step EACH PART CLEARLY please thanks!! im reposting this please solve all parts and drawit not just word explanations!!arrow_forwardHi!! Please provide a solution that is handwritten. this is an inorganic chemistry question please answer accordindly!! its just one question with parts JUST ONE QUESTION, please answer EACH part PART A AND PART B!!!!! till the end and dont just provide wordy explanations wherever asked for structures, please DRAW DRAW them on a paper and post clearly!! answer the full question with all details EACH PART CLEARLY please thanks!! im reposting this please solve all parts and drawit not just word explanations!!arrow_forwardHi!! Please provide a solution that is handwritten. this is an inorganic chemistry question please answer accordindly!! its just one question with parts JUST ONE QUESTION, please answer EACH part till the end and dont just provide wordy explanations wherever asked for structures, please DRAW DRAW them on a paper and post clearly!! answer the full question with all details EACH PART CLEARLY please thanks!! im reposting this please solve all parts and drawit not just word explanations!!arrow_forward
- 8b. Explain, using key intermediates, why the above two products are formed instead of the 1,2-and 1,4- products shown in the reaction below. CIarrow_forward(5pts) Provide the complete arrow pushing mechanism for the chemical transformation depicted below Use proper curved arrow notation that explicitly illustrates all bonds being broken, and all bonds formed in the transformation. Also, be sure to include all lone pairs and formal charges on all atoms involved in the flow of electrons. CH3O H I I CH3O-H H I ① Harrow_forward6. Draw the products) formed from the following reactions. (a) HIarrow_forward
- Don't used Ai solutionarrow_forwardPlease correct answer and don't used hand raitingarrow_forward1. For each of the following, predict the products of the reaction by writing a balance net ionic equation for each. If no reaction is expected, then write NO REACTION. (a) AgNO3 (aq) is mixed with Na2CO3 (aq). (b) An aqueous solution of ammonium sulfate is added to an aqueous solution of calcium chloride. (c) RbI (aq) is added to Pb(NO3)2 (aq). (d) NaCl (s) is added to AgNO3 (aq).arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





