
Concept explainers
Which of the following pairs represent structural isomers, and which are simply the same compound?
a. 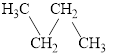 and
and
b. 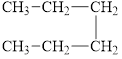 and
and
c. 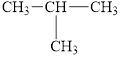 and
and
d. 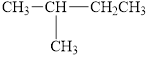 and
and 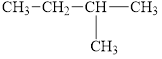
Trending nowThis is a popular solution!

Chapter 11 Solutions
Chemistry for Today: General, Organic, and Biochemistry
- Consider the following starting material and choose all of the functional groups that are likely to oxidize in it. a. aldehyde b. secondary alcohol c. alkane d. hemiacetal e. carboxylic acid f. alkenearrow_forwardWhich statement of the following is false? A. Cyclohexene has the general formula CH20-2 B. Alkenes are also referred to as olefins O C. The alkene C atoms are sp2 hybridized and have a bond angle of 120°C D. Trans isomers are identical to (Z) isomers E. The bond angle around the alkyne C atoms is 180°Carrow_forwardA saturated hydrocarbon Y with molecular formula of C4H8 exists as a pair of cis andtrans isomers:a. a. Draw the structural formulae for both cis and trans isomersb. b. Explain why Y exhibits geometrical isomersarrow_forward
- The two molecules represented below are examples of ____. CH3–CH2–O–CH2CH3 CH3CH2CH2CH2–OH Select one: A. stereoisomers B. geometric isomers C. optical isomers D. structural isomersarrow_forward1.Draw the structural formula of CH 3 CH(CH 3 )(CH 2 ) 4 CH3 2. How does the general formula of a cycloalkane compare to that of an alkane?arrow_forward1. what grouo does the ff organic compound belong? a. ketone b. ether c. cyloalkane d. esther 2. what group does the ff organic compound belong? a. amide b. azo c. nitrile d. amine 3. what is the priority functional group of the ff organic compound? a. carboxyl b. hydroxyl c. carbonyl d. hydroxidearrow_forward
- 1. Which of the following families of organic compounds share the same general molecular formula with normal chain alkynes? a. Alkanes and alkenes b. Alkanes and bicycloalkanes c. Cycloalkenes and bicycloalkanes d. Cycloalkenes and alkenes e. Cycloalkanes and cycloalkenesarrow_forwardA. A,C, and B D B. A and D only C. A, B, and C, D D. A, D and B, C E. C and D onlyarrow_forwardWhat is the condensed structural formula for 3,3-diethyl-2-methylhexane? A. CH3CH2CH(CH2CH3)CH2(CH3)CH2CH3 B. CH3CH2C(CH2CH3)2CH2(CH3)CH2CH3 C. CH3CH(CH3)C(CH2CH3)2CH2CH2CH3 D. CH3C(CH3)2C(CH2CH3)2CH2CH2CH3arrow_forward
- Hexane and cyclohexane are examples of two molecules that are. A . Constitutional Isomers B. cis - trans isomers C. identical D. enanitiomers E . Unrelated with different formulasarrow_forwardWhat kind of isomerism is involved?arrow_forward1. What functional group is produced when an aldehyde reacts with H2/Pt? A.secondary alcohol B. carboxylic acid C.hemiacetal D. primary alcohol E.alkane F.tertiary alcohol G. alkene 2. What reaction occurs when an aldehyde reacts with H2/Pt to form a primary alcohol? A. Hydration B. Hydration C. Dehydration D. Oxidation E. Reduction( hydrogentation) 3. What reaction occurs when an Ester react with H+/H2O to from a carboxylic acid and alcohol? A. Dehydration B. Reduction ( Hydrogenation) C.Hydrolysis D. Hydration E.oxidationarrow_forward

 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





