
Concept explainers
(a)
Interpretation:
The structural formula of the given compound is to be predicted.
Concept introduction:
Those compounds which have the same molecular formula but have different arrangements of atoms are known as isomers. The phenomenon is called as isomerism. The isomers are generally classified as structural isomers and stereoisomers. Stereoisomers are further divided into two categories diastereomers and enantiomers.
Answer to Problem 11.55E
The structural formula of the given compound is shown below as,
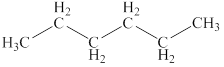
Explanation of Solution
The given compound is
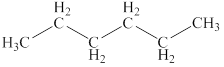
Figure 1
The structural formula of the given compound is shown in Figure 1.
(b)
Interpretation:
The structural formula of the given compound is to be predicted.
Concept introduction:
Those compounds which have the same molecular formula but have different arrangements of atoms are known as isomers. The phenomenon is called as isomerism. The isomers are generally classified as structural isomers and stereoisomers. Stereoisomers are further divided into two categories diastereomers and enantiomers.
Answer to Problem 11.55E
The structural formulas of the given compound are shown below as,
Explanation of Solution
The given compound is

Figure 2
The structural formulas of the given compound are shown in Figure 2.
(c)
Interpretation:
The structural formula of the given compound is to be predicted.
Concept introduction:
Those compounds which have the same molecular formula but have different arrangements of atoms are known as isomers. The phenomenon is called as isomerism. The isomers are generally classified as structural isomers and stereoisomers. Stereoisomers are further divided into two categories diastereomers and enantiomers.
Answer to Problem 11.55E
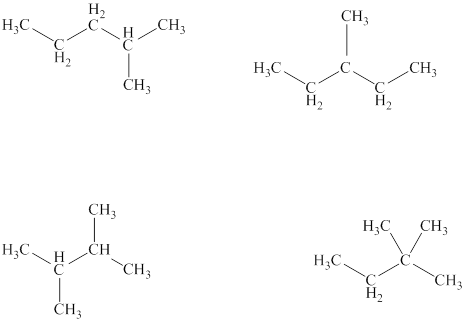
The structural formulas of the given compound are shown below as,
Explanation of Solution
Conformers are the type of isomers that can be interconverted into one other by simple rotation of bond.
The given compound is

Figure 3
The structural formulas of the given compound are shown in Figure 3.
(d)
Interpretation:
The structural formula of the given compound is to be predicted.
Concept introduction:
Those compounds which have the same molecular formula but have different arrangements of atoms are known as isomers. The phenomenon is called as isomerism. The isomers are generally classified as structural isomers and stereoisomers. Stereoisomers are further divided into two categories diastereomers and enantiomers.
Answer to Problem 11.55E
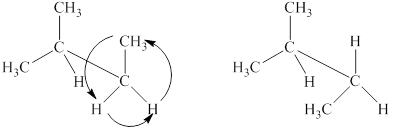
The structural formulas of the given compound are shown below as,
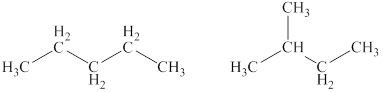
Explanation of Solution
The given compound,
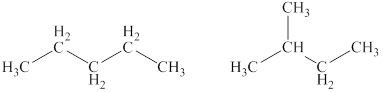
Figure 4
The structural formulas of the given compound are shown in Figure 4.
(e)
Interpretation:
The structural formula of the given compound is to be predicted.
Concept introduction:
Those compounds which have the same molecular formula but have different arrangements of atoms are known as isomers. The phenomenon is called as isomerism. The isomers are generally classified as structural isomers and stereoisomers. Stereoisomers are further divided into two categories diastereomers and enantiomers.
Answer to Problem 11.55E
The structural formula of the given compound is shown below as,

Explanation of Solution
The given compound is

Figure 5
The structural formula of the given compound is shown in Figure 5.
(f)
Interpretation:
The structural formula of the given compound is to be predicted.
Concept introduction:
Those compounds which have the same molecular formula but have different arrangements of atoms are known as isomers. The phenomenon is called as isomerism. The isomers are generally classified as structural isomers and stereoisomers. Stereoisomers are further divided into two categories diastereomers and enantiomers.
Answer to Problem 11.55E
The structural formulas of the given compound are shown below as,
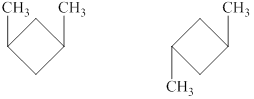
Explanation of Solution
The given compound,

Figure 6
The structural formulas of the given compound are shown in Figure 6.
(g)
Interpretation:
The structural formula of the given compound is to be predicted.
Concept introduction:
Those compounds which have the same molecular formula but have different arrangements of atoms are known as isomers. The phenomenon is called as isomerism. The isomers are generally classified as structural isomers and stereoisomers. Stereoisomers are further divided into two categories diastereomers and enantiomers.
Answer to Problem 11.55E
The structural formula of the given compound is shown below as,
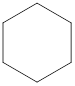
Explanation of Solution
The given compound,

Figure 7
The structural formula of the given compound is shown in Figure 7.
Want to see more full solutions like this?
Chapter 11 Solutions
Chemistry for Today: General, Organic, and Biochemistry
- 4. Refer to the data below to answer the following questions: The octapeptide saralasin is a specific antagonist of angiotensin II. A derivative of saralasin is used therapeutically as an antihypertensive. Amino acid analysis of saralasin show the presence of the following amino acids: Ala, Arg, His, Pro, Sar, Tyr, Val, Val A.Sar is the abbreviation for sarcosine, N-methyl aminoethanoic acid. Draw the structure of sarcosine. B. N-Terminal analysis by the Edman method shows saralasin contains sarcosine at the N-terminus. Partial hydrolysis of saralasin with dilute hydrochloric acid yields the following fragments: Tyr-Val-His Sar-Arg-Val His-Pro-Ala Val-Tyr-Val Arg-Val-Tyr What is the structure of saralasin?arrow_forwardWhat is the structure of the DNA backbone?arrow_forwardPLEASE PLEASE PLEASE use hand drawn structures when possarrow_forward
- . M 1- MATCH each of the following terms to a structure from the list below. There is only one correct structure for each term and structures may be used more than once. Place the letter of the structure in the blank to the left of the corresponding term. A. Sanger dideoxy method C. Watson-Crick B. GAUCGUAAA D. translation E. HOH2C OH OH G. transcription I. AUGGCUGAG 0 K. OPOH2C 0- OH N- H NH2 F. -OPOH2C 0- OH OH H. Maxam-Gilbert method J. replication N L. HOH2C a. b. C. d. e. f. g. B M. AGATCGCTC a pyrimidine nucleoside RNA base sequence with guanine at the 3' end. DNA base sequence with cytosine at the 3' end. a purine nucleoside DNA sequencing method for the human genome 2'-deoxyadenosine 5'-phosphate process by which mRNA directs protein synthesis OH NH2arrow_forwardPlease use hand drawn structures when neededarrow_forwardB. Classify the following amino acid. Atoms other than carbon and hydrogen are labeled. a. acidic b. basic C. neutral C. Consider the following image. Which level of protein structure is shown here? a. primary b. secondary c. tertiary d. quaternary D. Consider the following image. H RH H HR H R HR HR RH Which level of protein structure is shown in the box? a. primary b. secondary R c. tertiary d. quaternary コー Rarrow_forward
- Briefly answer three from the followings: a. What are the four structures of the protein? b. Why is the side chain (R) attached to the alpha carbon in the amino acids is important for the function? c. What are the types of amino acids? And how is it depend on the (R) structure? d. Write a reaction to prepare an amino acid. prodarrow_forwardAnswe Answer A and B pleasearrow_forward3. Refer to the data below to answer the following questions: Isoelectric point Amino Acid Arginine 10.76 Glutamic Acid 3.22 Tryptophan 5.89 A. Define isoelectric point. B. The most basic amino acid is C. The most acidic amino acid is sidizo zoarrow_forward
- 3. A gas mixture contains 50 mol% H2 and 50 mol% He. 1.00-L samples of this gas mixture are mixed with variable volumes of O2 (at 0 °C and 1 atm). A spark is introduced to allow the mixture to undergo complete combustion. The final volume is measured at 0 °C and 1 atm. Which graph best depicts the final volume as a function of the volume of added O2? (A) 2.00 1.75 Final Volume, L 1.50 1.25 1.00 0.75 0.50 0.25 0.00 0.00 0.25 0.50 2.00 (B) 1.75 1.50 Final Volume, L 1.25 1.00 0.75 0.50- 0.25 0.00 0.75 1.00 0.00 0.25 Volume O₂ added, L 2 0.50 0.75 1.00 Volume O₂ added, L 2 2.00 2.00 (C) (D) 1.75 1.75 1.50 1.50 Final Volume, L 1.25 1.00 0.75 0.50 Final Volume, L 1.25 1.00 0.75 0.50 0.25 0.25 0.00 0.00 0.00 0.25 0.50 0.75 1.00 0.00 0.25 Volume O₂ added, L 0.50 0.75 1.00 Volume O₂ added, L 2arrow_forwardLeucine is an essential amino acid with the systematic name 2-amino-3-methylpentanoic acid. It has pai 2.36 and pKa2 = 9.60. H2N-C(R)H-COOH and R is -CH2-CH(CH3)2 A. Draw the condensed structure for leucine, and label all chirality centers with an asterisk. B. How many possible stereoisomers of leucine are there? C. Draw a Fischer projection of L-leucine and label the chirality center(s) as R or S. D. What is the p/ of leucine? E. Draw the structure of the predominant form of leucine at 10.00. F. Draw the structure of the predominant form of leucine at pH = 1.50. G. Leucine is described as an essential amino acid. What does this mean? H. Show the alkyl halide you would use to prepare leucine by the amidomalonate method. =arrow_forwarda) Write out 6 completely different reactions of acetophenone (reagent, product). b) Write out 3 preparations of 1-methylcyclohexanol, using a different starting material for each one. You may use preps where you just change the functional group, and/or preps where you construct the carbon chain. c) Write out 3 preparations of 2-ethoxybenzoic acid, a different starting material for each one. You may use preps where you just change the functional group, and/or preps where you construct the carbon chain.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





