
Concept explainers
The following names are incorrect, according to IUPAC rules. Draw the structural formulas and tell why each name is incorrect. Write the correct name for each compound.
a.
b.
c.
d.
(a)
Interpretation:
The structural formulas for given compound is to be drawn. The reason as to why the given compound name is incorrect is to be stated. The correct name for the given compound is to be stated.
Concept introduction:
The condensed structural formula of the compound represents the arrangement of atoms by showing specific covalent bonds. The molecular formula represents the atoms and their subscript represents the number of atoms without showing any covalent bonds. The structural formula represents the arrangement of atoms in space.
Answer to Problem 11.42E
The structural formulas for given compound is,
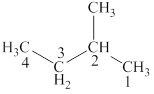
The longest chain in the given compound has four carbon atoms and one methyl group is attached to the second carbon. The correct IUPAC name of the given compound is
Explanation of Solution
The given compound is
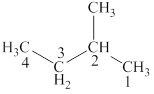
Figure 1
The structural formulas for given compound is shown in Figure 1. The longest chain in the given compound has four carbon atoms and one methyl group is attached to the second carbon. The correct IUPAC name of the given compound is
(b)
Interpretation:
The structural formulas for given compound is to be drawn. The reason as to why the given compound name is incorrect is to be stated. The correct name for the given compound is to be stated.
Concept introduction:
The condensed structural formula of the compound represents the arrangement of atoms by showing specific covalent bonds. The molecular formula represents the atoms and their subscript represents the number of atoms without showing any covalent bonds. The structural formula represents the arrangement of atoms in space.
Answer to Problem 11.42E
The structural formulas for given compound is,
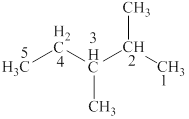
The lowest possible number is given to the carbon at which a group is attached. Thus, the correct IUPAC name of the given compound is
Explanation of Solution
The given compound is
The lowest possible number is given to the carbon from which a group is attached. Thus, the correct IUPAC name of the given compound is

Figure 2
The structural formulas for given compound is shown in Figure 2. The lowest possible number is given to the carbon from which a group is attached. Thus, the correct IUPAC name of the given compound is
(c)
Interpretation:
The structural formulas for given compound is to be drawn. The reason as to why the given compound name is incorrect is to be stated. The correct name for the given compound is to be stated.
Concept introduction:
The condensed structural formula of the compound represents the arrangement of atoms by showing specific covalent bonds. The molecular formula represents the atoms and their subscript represents the number of atoms without showing any covalent bonds. The structural formula represents the arrangement of atoms in space.
Answer to Problem 11.42E
The structural formulas for given compound is,
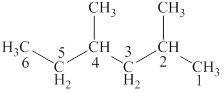
The parent chain is hexane and one methyl group each is attached to second and fourth carbon atom. Thus, the correct IUPAC name of the given compound is
Explanation of Solution
The given compound is
The longest chain in the given compound has six carbon atoms. Thus, the parent chain is hexane. The lowest possible number is given to the carbon from which a group is attached. Thus, methyl group are attached to second and fourth carbon. Thus, the correct IUPAC name of the given compound is

Figure 3
The structural formulas for given compound is shown in Figure 3. The parent chain is hexane and one methyl group each is attached to second and fourth carbon atoms. Thus, the correct IUPAC name of the given compound is
(d)
Interpretation:
The structural formulas for given compound is to be drawn. The reason as to why the given compound name is incorrect is to be stated. The correct name for the given compound is to be stated.
Concept introduction:
The condensed structural formula of the compound represents the arrangement of atoms by showing specific covalent bonds. The molecular formula represents the atoms and their subscript represents the number of atoms without showing any covalent bonds. The structural formula represents the arrangement of atoms in space.
Answer to Problem 11.42E
The structural formulas for given compound is,
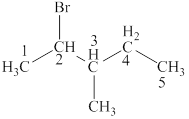
The parent chain is pentane and bromine and methyl group are attached to second and third carbon respectively. Thus, the correct IUPAC name of the given compound is
Explanation of Solution
The given compound is
The longest chain in the given compound has five carbon atoms. Thus, the parent chain is pentane. The lowest possible number is given to the carbon from which a group is attached. Thus, bromine and methyl group are attached to second and third carbon respectively. Thus, the correct IUPAC name of the given compound is

Figure 4
The structural formulas for given compound is shown in Figure 4. The parent chain ispentane and bromine and methyl group are attached to second and third carbon respectively. Thus, the correct IUPAC name of the given compound is
Want to see more full solutions like this?
Chapter 11 Solutions
Chemistry for Today: General, Organic, and Biochemistry
- Synthesize the following:arrow_forwardDid you report your data to the correct number of significant figures? Temperature of cold water (°C) 4.0 Temperature of hot water ("C) 87.0 Volume of cold water (mL) 94.0 Volume of hot water (mL) 78.0 Final temperature after mixing ("C) 41.0 Mass of cold water (g) 94.0 Mass of hot water (g) 78.0 Calorimeter constant (J/°C) 12.44 How to calculate the calorimeter constantarrow_forwardplease draw the arrowsarrow_forward
- Part 1. Draw monomer units of the following products and draw their reaction mechanism (with arrow pushing) Temporary cross-linked polymer Using: 4% polyvinyl alcohol+ methyl red + 4% sodium boratearrow_forwardcan you please answer both these questions and draw the neccesaryarrow_forwardcan you please give the answer for both these pictures. thankyouarrow_forward
- Part 1. Draw monomer units of the following products and draw their reaction mechanism (with arrow pushing) | Bakelite like polymer Using: Resorcinol + NaOH + Formalinarrow_forwardQuestion 19 0/2 pts 3 Details You have a mixture of sodium chloride (NaCl) and potassium chloride (KCl) dissolved in water and want to separate out the Cl- ions by precipitating them out using silver ions (Ag+). The chemical equation for the net ionic reaction of NaCl and KCl with silver nitrate, AgNO3, is shown below. Ag+(aq) + Cl(aq) → AgCl(s) The total mass of the NaCl/KCl mixture is 1.299 g. Adding 50.42 mL of 0.381 M solution precipitates out all of the Cl-. What are the masses of NaCl and KCl in the mixture? Atomic masses: g: Mass of NaCl g: Mass of KCL Ag = 107.868 g mol- 1 Cl = 35.453 g mol- 1 K = 39.098 g mol- N = 14.007 g mol−1 Na = 22.99 g mol−1 0 = 15.999 g mol 1 Question Help: ✓ Message instructor Submit Questionarrow_forwardPart 1. Draw monomer units of the following products and draw their reaction mechanism (with arrow pushing) Polyester fiber Using a) pthalic anhydride + anhydrous sodium acetate + ethylene glycol B)pthalic anhydride + anhydrous sodium acetate + glycerolarrow_forward
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning





