
Concept explainers
Classify each of the following compounds as a normal
a. 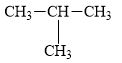
b. 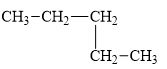
c. 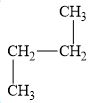
d. 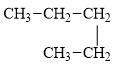
e. 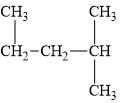
f. 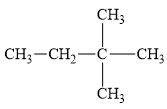
(a)
Interpretation:
The alkane
Concept introduction:
Alkanes are acyclic saturated hydrocarbons. They are also known as the paraffin. The alkanes contain singly bonded carbon atoms and hydrogen atoms. The alkanes have general formula
Answer to Problem 11.28E
The compound,
Explanation of Solution
The alkane is shown below.
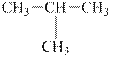
Figure 1
Branched alkanes do not have linear structure and they have alkyl substituents. In the above compound,
The compound,
(b)
Interpretation:
The alkane pentane is to be classified as normal alkane or a branched alkane.
Concept introduction:
Alkanes are acyclic saturated hydrocarbons. They are also known as the paraffin. The alkanes contain singly bonded carbon atoms and hydrogen atoms. The alkanes have general formula
Answer to Problem 11.28E
The compound, pentane is a normal alkane.
Explanation of Solution
The alkane is shown below.
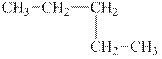
Figure 2
Normal alkanes have linear structure and have no alkyl substituents attached to it. The above compound, pentane has linear structure and has no alkyl substituents. Therefore, it is a normal alkane.
The compound, pentane is a normal alkane.
(c)
Interpretation:
The alkane butane is to be classified as normal alkane or a branched alkane.
Concept introduction:
Alkanes are acyclic saturated hydrocarbons. They are also known as the paraffin. The alkanes contain singly bonded carbon atoms and hydrogen atoms. The alkanes have general formula
Answer to Problem 11.28E
The compound, butane is a normal alkane.
Explanation of Solution
The alkane is shown below.
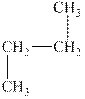
Figure 3
Normal alkanes have linear structure and have no alkyl substituents attached to it. The above compound, butane has linear structure and has no alkyl substituents. Therefore, it is a normal alkane.
The compound, butane is a normal alkane.
(d)
Interpretation:
The alkane pentane is to be classified as normal alkane or a branched alkane.
Concept introduction:
Alkanes are acyclic saturated hydrocarbons. They are also known as the paraffin. The alkanes contain singly bonded carbon atoms and hydrogen atoms. The alkanes have general formula
Answer to Problem 11.28E
The compound, pentane is a normal alkane.
Explanation of Solution
The alkane is shown below.
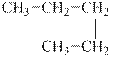
Figure 4
Normal alkanes have linear structure and have no alkyl substituents attached to it. The above compound, pentane has linear structure and has no alkyl substituents. Therefore, it is a normal alkane.
The compound, pentane is a normal alkane.
(e)
Interpretation:
The alkane
Concept introduction:
Alkanes are acyclic saturated hydrocarbons. They are also known as the paraffin. The alkanes contain singly bonded carbon atoms and hydrogen atoms. The alkanes have general formula
Answer to Problem 11.28E
The compound,
Explanation of Solution
The alkane is shown below.
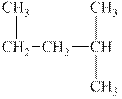
Figure 5
Branched alkanes do not have linear structure and they have alkyl substituents. In above compound,
The compound,
(f)
Interpretation:
The alkane
Concept introduction:
Alkanes are acyclic saturated hydrocarbons. They are also known as the paraffin. The alkanes contain singly bonded carbon atoms and hydrogen atoms. The alkanes have general formula
Answer to Problem 11.28E
The compound,
Explanation of Solution
The alkane is shown below.
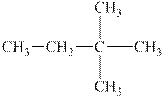
Figure 6
Branched alkanes do not have linear structure and they have alkyl substituents. In the above compound,
The compound,
Want to see more full solutions like this?
Chapter 11 Solutions
Chemistry for Today: General, Organic, and Biochemistry
- Pleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardThe Ksp for lead iodide ( Pbl₂) is 1.4 × 10-8. Calculate the solubility of lead iodide in each of the following. a. water Solubility = mol/L b. 0.17 M Pb(NO3)2 Solubility = c. 0.017 M NaI mol/L Solubility = mol/Larrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning  Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





