
Organic Chemistry
4th Edition
ISBN: 9780073402772
Author: Janice G. Smith
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Question
Chapter 11, Problem 11.41P
Interpretation Introduction
Interpretation:
To determine two different
Concept introduction:
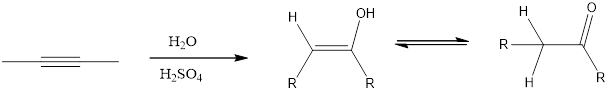
Addition of H2O to an alkyne resembles similar to the acid-catalyzed addition of H2O to an
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
>
You are trying to decide if there is a single reagent you can add that will make the following synthesis possible without any other
major side products:
1. ☑
CI
2. H3O+
O
Draw the missing reagent X you think will make this synthesis work in the drawing area below.
If there is no reagent that will make your desired product in good yield or without complications, just check the box under the
drawing area and leave it blank.
Click and drag to start drawing a
structure.
Explanation
Check
?
DO
18
Ar
B
© 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility
Don't use ai to answer I will report you answer
Consider a solution of 0.00304 moles of 4-nitrobenzoic acid (pKa = 3.442) dissolved in 25 mL water and titrated with 0.0991 M NaOH. Calculate the pH at the equivalence point
Chapter 11 Solutions
Organic Chemistry
Ch. 11 - Draw structures for the three alkynes having...Ch. 11 - Prob. 11.2PCh. 11 - Give the IUPAC name for each compound.Ch. 11 - Give the structures corresponding to each of the...Ch. 11 - Prob. 11.5PCh. 11 - Prob. 11.6PCh. 11 - Which bases can deprotonate acetylene? The pKa...Ch. 11 - Draw the organic products formed when each alkyne...Ch. 11 - Prob. 11.9PCh. 11 - Problem 11.9 Draw the products formed when is...
Ch. 11 - Explain the following result. Although alkenes...Ch. 11 - Problem 11.11 Draw the keto tautomer of each...Ch. 11 - Prob. 11.13PCh. 11 - a Draw two different enol tautomers of...Ch. 11 - Prob. 11.15PCh. 11 - Prob. 11.16PCh. 11 - Prob. 11.17PCh. 11 - Problem. 11.17 Show how , and can be used to...Ch. 11 - Prob. 11.19PCh. 11 - Draw the products of each reaction. a. b.Ch. 11 - Prob. 11.21PCh. 11 - Problem 11.21 Use retrosynthetic analysis to show...Ch. 11 - Prob. 11.23PCh. 11 - Give the IUPAC name for each compound. a. b.Ch. 11 - Prob. 11.25PCh. 11 - 11.25 Answer the following questions about...Ch. 11 - Prob. 11.27PCh. 11 - Give the IUPAC name for each alkyne.Ch. 11 - Prob. 11.29PCh. 11 - Which of the following pairs of compounds...Ch. 11 - Prob. 11.31PCh. 11 - 11.30 How is each compound related to A? Choose...Ch. 11 - Prob. 11.33PCh. 11 - Prob. 11.34PCh. 11 - Prob. 11.35PCh. 11 - 11.33 Draw the products formed when is treated...Ch. 11 - Draw the products formed when 3-hexyne is treated...Ch. 11 - Prob. 11.38PCh. 11 - Prob. 11.39PCh. 11 - What alkynes give each of the following ketones as...Ch. 11 - Prob. 11.41PCh. 11 - 11.37 What alkyne gives each compound as the only...Ch. 11 - Prob. 11.43PCh. 11 - Draw the structure of compounds A-E in the...Ch. 11 - Prob. 11.45PCh. 11 - Prob. 11.46PCh. 11 - 11.42 What reactions are needed to convert alcohol...Ch. 11 - Prob. 11.48PCh. 11 - Prob. 11.49PCh. 11 - 11.45 Explain the following statement. Although ...Ch. 11 - Draw a stepwise mechanism for the following...Ch. 11 - Prob. 11.52PCh. 11 - Prob. 11.53PCh. 11 - Prob. 11.54PCh. 11 - Prob. 11.55PCh. 11 - Synthesize each compound from acetylene. You may...Ch. 11 - Prob. 11.57PCh. 11 - Prob. 11.58PCh. 11 - 11.55 Devise a synthesis of the ketone, , from ...Ch. 11 - 11.56 Devise a synthesis of each compound using ...Ch. 11 - Prob. 11.61PCh. 11 - Prob. 11.62PCh. 11 - 11.60 Draw a stepwise mechanism for the following...Ch. 11 - Draw a stepwise mechanism for the following...Ch. 11 - Prob. 11.65PCh. 11 - Write a stepwise mechanism for each of the...Ch. 11 - Prob. 11.67PCh. 11 - 11.65 Explain why an optically active solution of ...
Knowledge Booster
Similar questions
- What is the name of the following compound? SiMe3arrow_forwardK Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
