
Organic Chemistry
4th Edition
ISBN: 9780073402772
Author: Janice G. Smith
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 11, Problem 11.12P
Draw the keto tautomer of each enol.
a. 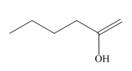 b.
b. 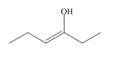 c.
c. 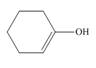
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
A solution containing 100.0 mL of 0.155 M EDTA buffered to pH 10.00 was titrated with 100.0 mL of 0.0152 M Hg(ClO4)2 in a cell:
calomel electrode (saturated)//titration solution/Hg(l)
Given the formation constant of Hg(EDTA)2-, logKf= 21.5, and alphaY4-=0.30, find out the cell voltage E.
Hg2+(aq) + 2e- = Hg(l) E0= 0.852 V
E' (calomel electrode, saturated KCl) = 0.241 V
From the following reduction potentials
I2 (s) + 2e- = 2I- (aq) E0= 0.535 V
I2 (aq) + 2e- = 2I- (aq) E0= 0.620 V
I3- (aq) + 2e- = 3I- (aq) E0= 0.535 V
a) Calculate the equilibrium constant for I2 (aq) + I- (aq) = I3- (aq).
b) Calculate the equilibrium constant for I2 (s) + I- (aq) = I3- (aq).
c) Calculate the solubility of I2 (s) in water.
2. (3 pts) Consider the unit cell for the spinel compound,
CrFe204. How many total particles are in the unit cell?
Also, show how the number of particles and their
positions are consistent with the CrFe204
stoichiometry - this may or may not be reflected by the
particle colors in the diagram. (HINT: In the diagram,
the blue particle is in an interior position while each red
particle is either in a corner or face position.)
Chapter 11 Solutions
Organic Chemistry
Ch. 11 - Draw structures for the three alkynes having...Ch. 11 - Prob. 11.2PCh. 11 - Give the IUPAC name for each compound.Ch. 11 - Give the structures corresponding to each of the...Ch. 11 - Prob. 11.5PCh. 11 - Prob. 11.6PCh. 11 - Which bases can deprotonate acetylene? The pKa...Ch. 11 - Draw the organic products formed when each alkyne...Ch. 11 - Prob. 11.9PCh. 11 - Problem 11.9 Draw the products formed when is...
Ch. 11 - Explain the following result. Although alkenes...Ch. 11 - Problem 11.11 Draw the keto tautomer of each...Ch. 11 - Prob. 11.13PCh. 11 - a Draw two different enol tautomers of...Ch. 11 - Prob. 11.15PCh. 11 - Prob. 11.16PCh. 11 - Prob. 11.17PCh. 11 - Problem. 11.17 Show how , and can be used to...Ch. 11 - Prob. 11.19PCh. 11 - Draw the products of each reaction. a. b.Ch. 11 - Prob. 11.21PCh. 11 - Problem 11.21 Use retrosynthetic analysis to show...Ch. 11 - Prob. 11.23PCh. 11 - Give the IUPAC name for each compound. a. b.Ch. 11 - Prob. 11.25PCh. 11 - 11.25 Answer the following questions about...Ch. 11 - Prob. 11.27PCh. 11 - Give the IUPAC name for each alkyne.Ch. 11 - Prob. 11.29PCh. 11 - Which of the following pairs of compounds...Ch. 11 - Prob. 11.31PCh. 11 - 11.30 How is each compound related to A? Choose...Ch. 11 - Prob. 11.33PCh. 11 - Prob. 11.34PCh. 11 - Prob. 11.35PCh. 11 - 11.33 Draw the products formed when is treated...Ch. 11 - Draw the products formed when 3-hexyne is treated...Ch. 11 - Prob. 11.38PCh. 11 - Prob. 11.39PCh. 11 - What alkynes give each of the following ketones as...Ch. 11 - Prob. 11.41PCh. 11 - 11.37 What alkyne gives each compound as the only...Ch. 11 - Prob. 11.43PCh. 11 - Draw the structure of compounds A-E in the...Ch. 11 - Prob. 11.45PCh. 11 - Prob. 11.46PCh. 11 - 11.42 What reactions are needed to convert alcohol...Ch. 11 - Prob. 11.48PCh. 11 - Prob. 11.49PCh. 11 - 11.45 Explain the following statement. Although ...Ch. 11 - Draw a stepwise mechanism for the following...Ch. 11 - Prob. 11.52PCh. 11 - Prob. 11.53PCh. 11 - Prob. 11.54PCh. 11 - Prob. 11.55PCh. 11 - Synthesize each compound from acetylene. You may...Ch. 11 - Prob. 11.57PCh. 11 - Prob. 11.58PCh. 11 - 11.55 Devise a synthesis of the ketone, , from ...Ch. 11 - 11.56 Devise a synthesis of each compound using ...Ch. 11 - Prob. 11.61PCh. 11 - Prob. 11.62PCh. 11 - 11.60 Draw a stepwise mechanism for the following...Ch. 11 - Draw a stepwise mechanism for the following...Ch. 11 - Prob. 11.65PCh. 11 - Write a stepwise mechanism for each of the...Ch. 11 - Prob. 11.67PCh. 11 - 11.65 Explain why an optically active solution of ...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Choose the best answer to each of the following. Explain your reasoning. If Earth were twice as far as it actua...
Cosmic Perspective Fundamentals
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
6. How can you use the features found in each chapter?
Human Anatomy & Physiology (2nd Edition)
Separate the list P,F,V,,T,a,m,L,t, and V into intensive properties, extensive properties, and nonproperties.
Fundamentals Of Thermodynamics
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- From the following potentials, calculate the activity of Cl- in saturated KCl. E0 (calomel electrode)= 0.268 V E (calomel electrode, saturated KCl)= 0.241 Varrow_forwardCalculate the voltage of each of the following cells. a) Fe(s)/Fe2+ (1.55 x 10-2 M)//Cu2+ (6.55 x 10-3 M)/Cu(s) b) Pt, H2 (0.255 bar)/HCl (4.55 x 10-4 M), AgCl (sat'd)/Ag Fe2+ +2e- = Fe E0= -0.44 V Cu2+ + 2e- = Cu E0= 0.337 V Ag+ + e- = Ag E0= 0.799 V AgCl(s) + e- = Ag(s) + Cl- E0= 0.222 V 2H+ + 2e- = H2 E0= 0.000 Varrow_forwardA solution contains 0.097 M Ce3+, 1.55x10-3 M Ce4+, 1.55x10-3 M Mn2+, 0.097 M MnO4-, and 1.00 M HClO4 (F= 9.649 x 104 C/mol). a) Write a balanced net reaction that can occur between species in this solution. b) Calculate deltaG0 and K for the reaction. c) Calculate E and deltaG for the conditions given. Ce4+ + e- = Ce3+ E0= 1.70 V MnO4- + 8H+ + 5e- = Mn2+ + 4H2O E0= 1.507 Varrow_forward
- 1. Provide a step-by-step mechanism for formation of ALL STEREOISOMERS in the following reaction. Na HCO3 (Sodium bicarbonate, baking soda) is not soluble in CH2Cl2. The powder is a weak base used to neutralize strong acid (pKa < 0) produced by the reaction. Redraw the product to show the configuration(s) that form at C-2 and C-4. Br2 OH CH2Cl2 Na* HCO3 Br HO OH + Na Br +arrow_forward2. Specify the solvent and reagent(s) required to carry out each of the following FGI. If two reagent sets must be used for the FGI, specify the solvent and reagent(s) for each reagent set. If a reaction cannot be carried out with reagents (sets) class, write NP (not possible) in the solvent box for reagent set #1. Use the letter abbreviation for each solvent; use a number abbreviation for reagent(s). Solvents: CH2Cl2 (A); H₂O (B); Reagents: HBr (1); R₂BH (6); H2SO4 (2); CH3OH (C); Br₂ (3); CH3CO₂H (D) NaHCO3 (4); Hg(OAc)2 (5); H₂O2/HO (7); NaBH4 (8) Reagent Set #1 Reagent Set #2 FGI + enant OH Solvent Reagent(s) Solvent Reagent(s)arrow_forwardGermanium (Ge) is a semiconductor with a bandgap of 2.2 eV. How could you dope Ge to make it a p-type semiconductor with a larger bandgap? Group of answer choices It is impossible to dope Ge and have this result in a larger bandgap. Dope the Ge with silicon (Si) Dope the Ge with gallium (Ga) Dope the Ge with phosphorus (P)arrow_forward
- Which of the following semiconductors would you choose to have photons with the longest possible wavelengths be able to promote electrons to the semiconductor's conduction band? Group of answer choices Si Ge InSb CdSarrow_forwardWhich of the following metals is the only one with all of its bands completely full? Group of answer choices K Na Ca Alarrow_forward2. Specify the solvent and reagent(s) required to carry out each of the following FGI. If two reagent sets must be used for the FGI, specify the solvent and reagent(s) for each reagent set. If a reaction cannot be carried out with reagents (sets) class, write NP (not possible) in the solvent box for reagent set #1. Use the letter abbreviation for each solvent; use a number abbreviation for reagent(s). Solvents: CH2Cl2 (A); Reagents: H₂O (B); CH3CO₂H (D) NaHCO3 (4); Hg(OAc)2 (5); HBr (1); R₂BH (6); H2SO4 (2); CH3OH (C); Br₂ (3); H₂O₂ / HO- (7); NaBH4 (8) Reagent Set #1 Reagent Set #2 FGI OH - α-α Br + enant Solvent Reagent(s) Solvent Reagent(s)arrow_forward
- Based on concepts from Lecture 3-5, which of the following ionic compounds should be most soluble in water? Group of answer choices MgO BeO CaO BaOarrow_forwardFrom an energy standpoint, which two process - in the correct order - are involved in the dissolving of an ionic compound crystal? Group of answer choices Water coordination to the ions followed by sublimation into the gas phase Sublimation of the crystal into gas-phase ions followed by water coordination to the ions Ion dissociation from the crystal followed by water coordination to the ions Water coordination to the ions followed by ion dissociation from the crystalarrow_forwardFor which Group 2 metal (M), is this process the most exothermic? M2+(g) + O2−(g) + CO2(g) → MO(s) + CO2(g) Group of answer choices M = Sr M = Mg M = Ca M = Baarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY