
Organic And Biological Chemistry
7th Edition
ISBN: 9781305081079
Author: STOKER, H. Stephen (howard Stephen)
Publisher: Cengage Learning,
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 11, Problem 11.3EP
Indicate whether each of the following pentoses is present in DNA molecules.
- a. Ribose
- b. 2-Deoxyribose
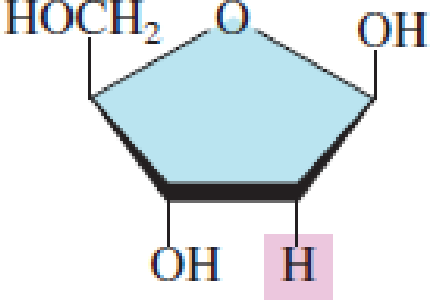
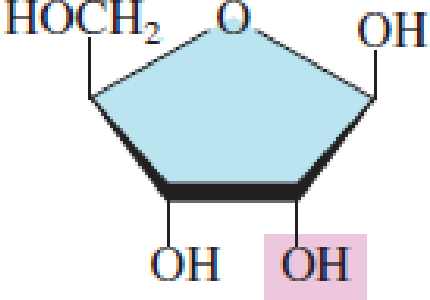
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Construct a molecular orbital diagram for carbon monoxide. Identify the relevant point group,include all of the appropriate symmetry labels and pictures, and fill in the electrons. Make sure toaccount for the difference in electronegativity between C and O. Hint: CO is substantiallyisoelectronic to N2. (PLEASE DRAW THE ENTIRE MO DIAGRAM!!!)
please help with hw
help me solve this hw
Chapter 11 Solutions
Organic And Biological Chemistry
Ch. 11.1 - Which of the following statements concerning...Ch. 11.1 - Which of the following statements concerning...Ch. 11.2 - Any given nucleotide in a nucleic acid contains a....Ch. 11.2 - How many different sugars and how many different...Ch. 11.2 - How many different heterocyclic bases that are...Ch. 11.3 - Which of the following is present in nucleotides...Ch. 11.3 - Prob. 2QQCh. 11.3 - Prob. 3QQCh. 11.3 - Prob. 4QQCh. 11.4 - Prob. 1QQ
Ch. 11.4 - Prob. 2QQCh. 11.4 - Prob. 3QQCh. 11.4 - In a segment of a nucleic acid a terminal...Ch. 11.4 - Prob. 5QQCh. 11.5 - Which of the following statements concerning a DNA...Ch. 11.5 - Prob. 2QQCh. 11.5 - Prob. 3QQCh. 11.5 - Prob. 4QQCh. 11.6 - Prob. 1QQCh. 11.6 - In DNA replication the DNA double helix unwinds...Ch. 11.6 - Prob. 3QQCh. 11.6 - Prob. 4QQCh. 11.6 - Prob. 5QQCh. 11.7 - Prob. 1QQCh. 11.7 - The product of the first phase of protein...Ch. 11.8 - Prob. 1QQCh. 11.8 - Prob. 2QQCh. 11.8 - Prob. 3QQCh. 11.8 - Prob. 4QQCh. 11.9 - Which of the following statements concerning the...Ch. 11.9 - What is the complementary hnRNA base sequence...Ch. 11.9 - The location in a DNA strand carrying the...Ch. 11.9 - Prob. 4QQCh. 11.9 - Which of the following types of RNA is not part of...Ch. 11.10 - Prob. 1QQCh. 11.10 - The genetic code is a listing that gives...Ch. 11.10 - Prob. 3QQCh. 11.10 - Without reference to the genetic code, which of...Ch. 11.11 - Which of the following is an incorrect pairing of...Ch. 11.11 - Prob. 2QQCh. 11.11 - A tRNA molecule with the anticodon 5 AAG 3 will...Ch. 11.12 - Which of the following statements about ribosomes...Ch. 11.12 - Prob. 2QQCh. 11.12 - Prob. 3QQCh. 11.12 - Prob. 4QQCh. 11.12 - Prob. 5QQCh. 11.13 - Which of the following describes the effect of a...Ch. 11.13 - Prob. 2QQCh. 11.13 - Which of the following statements applies to both...Ch. 11.14 - Prob. 1QQCh. 11.14 - Prob. 2QQCh. 11.15 - Prob. 1QQCh. 11.15 - Prob. 2QQCh. 11.15 - The role of E. coli plasmids in obtaining rDNA is...Ch. 11.15 - Prob. 4QQCh. 11.15 - Prob. 5QQCh. 11.16 - Prob. 1QQCh. 11.16 - Prob. 2QQCh. 11 - Indicate whether each of the following statements...Ch. 11 - Indicate whether each of the following statements...Ch. 11 - Indicate whether each of the following pentoses is...Ch. 11 - Indicate whether each of the pentoses in Problem...Ch. 11 - With the help of Figure 22-2, identify each of the...Ch. 11 - With the help of Figure 22-2, identify each of the...Ch. 11 - With the help of Figure 22-2, what is the...Ch. 11 - With the help of Figure 22-2, what is the...Ch. 11 - With the help of Figure 22-2, indicate whether...Ch. 11 - With the help of Figure 22-2, indicate whether...Ch. 11 - Prob. 11.11EPCh. 11 - How many different choices are there for each of...Ch. 11 - Indicate whether each of the following statements...Ch. 11 - Prob. 11.14EPCh. 11 - What is the name of the nucleoside that contains...Ch. 11 - What is the name of the nucleoside that contains...Ch. 11 - Indicate whether each of the following statements...Ch. 11 - Indicate whether each of the following statements...Ch. 11 - Indicate whether each of the following is a DNA...Ch. 11 - Indicate whether each of the following is a DNA...Ch. 11 - Prob. 11.21EPCh. 11 - Prob. 11.22EPCh. 11 - What nitrogen-containing base and what sugar are...Ch. 11 - What nitrogen-containing base and what sugar are...Ch. 11 - Prob. 11.25EPCh. 11 - Prob. 11.26EPCh. 11 - Consider the following nucleotide. a. What is the...Ch. 11 - Consider the following nucleotide. a. What is the...Ch. 11 - Prob. 11.29EPCh. 11 - Prob. 11.30EPCh. 11 - For the trinucleotide 5 GCA 3 a. How many...Ch. 11 - For the trinucleotide 5 UCG 3 a. How many...Ch. 11 - Is the trinucleotide in Problem 22-31 found only...Ch. 11 - Is the trinucleotide in Problem 22-32 found only...Ch. 11 - In the lengthening of a polynucleotide chain,...Ch. 11 - Prob. 11.36EPCh. 11 - Draw the structure of the RNA dinucleotide 5 UG 3.Ch. 11 - Draw the structure of the DNA dinucleotide 5 TA 3.Ch. 11 - For the trinucleotide 5 T-G-A 3 a. How many...Ch. 11 - For the trinucleotide 5 U-C-G 3 a. How many...Ch. 11 - Prob. 11.41EPCh. 11 - Prob. 11.42EPCh. 11 - Prob. 11.43EPCh. 11 - Prob. 11.44EPCh. 11 - The base content of a particular DNA molecule is...Ch. 11 - The base content of a particular DNA molecule is...Ch. 11 - What structural consideration prevents the bases A...Ch. 11 - What structural consideration prevents the bases C...Ch. 11 - The base composition for one of the strands of a...Ch. 11 - The base composition for one of the strands of a...Ch. 11 - Convert each of the following 3-to-5 DNA base...Ch. 11 - Convert each of the following 3-to-5 DNA base...Ch. 11 - Using the concept of complementary base pairing,...Ch. 11 - Using the concept of complementary base pairing,...Ch. 11 - For the DNA segment 5 TTGCAC 3 how many of each of...Ch. 11 - For the DNA segment 5 TAGATG 3 how many of each of...Ch. 11 - What is the base sequence, specified in the 5-to-3...Ch. 11 - What is the base sequence, specified in the 5-to-3...Ch. 11 - In the replication of a DNA molecule, two daughter...Ch. 11 - In the replication of a DNA molecule, two daughter...Ch. 11 - How does the synthesis of a daughter DNA strand...Ch. 11 - In the context of DNA replication, what is the...Ch. 11 - Prob. 11.63EPCh. 11 - Prob. 11.64EPCh. 11 - Prob. 11.65EPCh. 11 - Prob. 11.66EPCh. 11 - Prob. 11.67EPCh. 11 - Prob. 11.68EPCh. 11 - Indicate whether each of the following statements...Ch. 11 - Prob. 11.70EPCh. 11 - Indicate whether each of the following statements...Ch. 11 - Prob. 11.72EPCh. 11 - Prob. 11.73EPCh. 11 - Prob. 11.74EPCh. 11 - Indicate whether the predominant cellular location...Ch. 11 - Indicate whether the predominant cellular location...Ch. 11 - Indicate whether each of the following situations...Ch. 11 - Indicate whether each of the following processes...Ch. 11 - Indicate whether each of the following statements...Ch. 11 - Indicate whether each of the following statements...Ch. 11 - Prob. 11.81EPCh. 11 - What is the base sequence, given in the 5-to-3...Ch. 11 - For each of the following DNA template strands,...Ch. 11 - For each of the following DNA template strands,...Ch. 11 - Prob. 11.85EPCh. 11 - Prob. 11.86EPCh. 11 - Prob. 11.87EPCh. 11 - Prob. 11.88EPCh. 11 - What mRNA base sequence, specified in the 5-to-3...Ch. 11 - Prob. 11.90EPCh. 11 - Prob. 11.91EPCh. 11 - What mRNA base sequence, specified in the 5-to-3...Ch. 11 - Prob. 11.93EPCh. 11 - Prob. 11.94EPCh. 11 - Prob. 11.95EPCh. 11 - Prob. 11.96EPCh. 11 - Prob. 11.97EPCh. 11 - Prob. 11.98EPCh. 11 - Prob. 11.99EPCh. 11 - Prob. 11.100EPCh. 11 - Prob. 11.101EPCh. 11 - Using the information in Table 22-2, determine...Ch. 11 - Using the information in Table 22-2, determine the...Ch. 11 - Prob. 11.104EPCh. 11 - Explain why the base sequence ATC could not be a...Ch. 11 - Explain why the base sequence AGAC could not be a...Ch. 11 - Prob. 11.107EPCh. 11 - Predict the sequence of amino acids coded by the...Ch. 11 - Prob. 11.109EPCh. 11 - Prob. 11.110EPCh. 11 - Determine each of the following items using the...Ch. 11 - Prob. 11.112EPCh. 11 - Prob. 11.113EPCh. 11 - Prob. 11.114EPCh. 11 - Prob. 11.115EPCh. 11 - Prob. 11.116EPCh. 11 - Prob. 11.117EPCh. 11 - Prob. 11.118EPCh. 11 - Which amino acid will a tRNA molecule be carrying...Ch. 11 - Prob. 11.120EPCh. 11 - Prob. 11.121EPCh. 11 - Identify the amino acid associated with each of...Ch. 11 - Prob. 11.123EPCh. 11 - Prob. 11.124EPCh. 11 - Indicate whether each of the following statements...Ch. 11 - Prob. 11.126EPCh. 11 - Prob. 11.127EPCh. 11 - Prob. 11.128EPCh. 11 - Prob. 11.129EPCh. 11 - Prob. 11.130EPCh. 11 - Prob. 11.131EPCh. 11 - Prob. 11.132EPCh. 11 - Which of these RNA types(1) mRNA, (2) hnRNA, (3)...Ch. 11 - Prob. 11.134EPCh. 11 - Consider the following mRNA base sequence 5CUUCAG3...Ch. 11 - Consider the following mRNA base sequence 5ACCCAC3...Ch. 11 - Consider the following DNA base sequence 3TTAATA5...Ch. 11 - Prob. 11.138EPCh. 11 - The DNA template strand segment 3TTCAAACCGTAC5...Ch. 11 - The DNA template strand segment 3TTCAAACCGTAC5...Ch. 11 - Prob. 11.141EPCh. 11 - Prob. 11.142EPCh. 11 - Prob. 11.143EPCh. 11 - Prob. 11.144EPCh. 11 - Prob. 11.145EPCh. 11 - Prob. 11.146EPCh. 11 - Prob. 11.147EPCh. 11 - Prob. 11.148EPCh. 11 - Prob. 11.149EPCh. 11 - Prob. 11.150EPCh. 11 - Prob. 11.151EPCh. 11 - Which of the following processes(1) transcription...Ch. 11 - Prob. 11.153EPCh. 11 - Prob. 11.154EP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Briefly explain chemical potential.arrow_forwardReason whether it is possible to determine changes in the Galvani potential difference at the metal-solution interface.arrow_forwardObtain the standard potential at 25°C of the Cu* I Cu | Pt electrode from the standard potentials E° Cu²+/Cu = 0.341 V and E Cu²+ /Cu+ = 0.153 V.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

World of Chemistry
Chemistry
ISBN:9780618562763
Author:Steven S. Zumdahl
Publisher:Houghton Mifflin College Div

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning
Nucleic acids - DNA and RNA structure; Author: MEDSimplified;https://www.youtube.com/watch?v=0lZRAShqft0;License: Standard YouTube License, CC-BY