
The gas-turbine portion of a combined gas–steam power plant has a pressure ratio of 16. Air enters the compressor at 300 K at a rate of 14 kg/s and is heated to 1500 K in the combustion chamber. The combustion gases leaving the gas turbine are used to heat the steam to 400°C at 10 MPa in a heat exchanger. The combustion gases leave the heat exchanger at 420 K. The steam leaving the turbine is condensed at 15 kPa. Assuming all the compression and expansion processes to be isentropic, determine (a) the mass flow rate of the steam, (b) the net power output, and (c) the thermal efficiency of the combined cycle. For air, assume constant specific heats at room temperature.
(a)
The mass flow rate of the steam.
Answer to Problem 77P
The mass flow rate of the steam is
Explanation of Solution
Show the
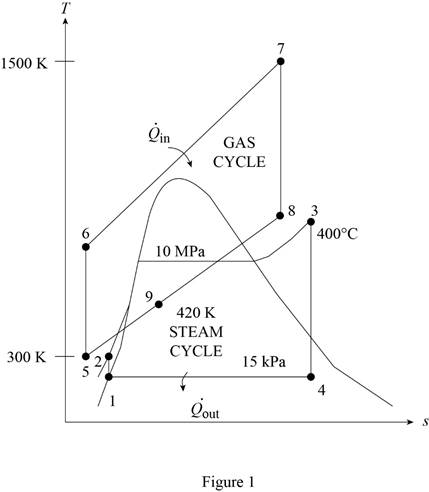
Determine the temperature of gas cycle at state 6.
Here, the temperature of gas cycle at state 5 is
Determine the rate of heat transfer into the gas turbine.
Here, the mass flow rate of air is
Determine the power rate for compressor of gas turbine.
Determine the temperature of gas cycle at state 8.
Here, the pressure of gas cycle at state 8 is
Determine the power rate for gas turbine of gas turbine.
Determine the net power output of the gas cycle.
Determine input work done per unit mass of the isentropic process for the steam cycle.
Here, the specific volume of the steam is
Determine the specific enthalpy at state 2 of the steam cycle.
Here, the specific enthalpy at the state 1 of the steam cycle is
Determine the quality at state 4 of the stream cycle.
Here, the specific entropy at state 4 is
Determine the specific enthalpy at state 4 of the steam cycle.
Here, the specific enthalpy of saturated liquid is
Write the expression for the steady-flow energy balance equation.
Here, the total energy rate of entering the system is
Substitute
Here, the temperature of gas cycle at state 8 is
Determine the power rate for gas turbine of steam cycle.
Here, the mass flow rate of the steam is
Determine the power rate of the isentropic process for the steam cycle.
Here, the mass flow rate of the steam is
Determine the net power output of the steam cycle.
Conclusion:
From the Table A-2, “Ideal-gas specific heats of various common gases”, obtain the value of specific heat of constant pressure and the ratio of specific heat at temperature of
Substitute 300 K for
Substitute
Substitute
Substitute 1500 K for
Substitute
Substitute 11547 kW for
From the Table A-4, “Saturated water-Pressure table”, obtain the value of the initial specific enthalpy at liquid state, specific volume at the liquid state, the specific entropy at liquid state, the specific enthalpy change upon vaporization at pressure, and the specific entropy change upon vaporization at pressure of 15 kPa as:
Substitute
Substitute
From the Table A-6, “Superheated water”, obtain the value of the specific enthalpy at state 3 and the specific entropy at state 3 at pressure of 10 MPa and temperature of
Substitute
Substitute 0.7528 for
Substitute
Thus, the mass flow rate of the steam is
Substitute
Substitute
Substitute 1384.013 kW for
(b)
The net work output of the combined cycle.
Answer to Problem 77P
The net work output of the combined cycle is
Explanation of Solution
Determine the net power output of combined cycle.
Conclusion:
Substitute 1371 kW for
Thus, the net work output of the combined cycle is
(c)
The thermal efficiency of the combined cycle.
Answer to Problem 77P
The thermal efficiency of the combined cycle is
Explanation of Solution
Determine the thermal efficiency of the combined cycle.
Conclusion:
Substitute 7819 kW for
Thus, the thermal efficiency of the combined cycle is
Want to see more full solutions like this?
Chapter 10 Solutions
Thermodynamics: An Engineering Approach
- Figure: 06_P041 Copyright 2013 Pearson Education, publishing a Prentice Hall 2. Determine the force that the jaws J of the metal cutters exert on the smooth cable C if 100-N forces are applied to the handles. The jaws are pinned at E and A, and D and B. There is also a pin at F. 400 mm 15° 20 mm A 15° 15 D B 30 mm² 80 mm 20 mm 400 mm Figure: 06_P090 Copyright 2013 Pearson Education, publishing as Prentice Hall 15° 100 N 100 N 15°arrow_forwardA telemetry system is used to quantify kinematic values of a ski jumper immediately before the jumper leaves the ramp. According to the system r=560 ft , r˙=−105 ft/s , r¨=−10 ft/s2 , θ=25° , θ˙=0.07 rad/s , θ¨=0.06 rad/s2 Determine the velocity of the skier immediately before leaving the jump. The velocity of the skier immediately before leaving the jump along with its direction is ? I have 112.08 ft/s but can't seem to get the direction correct. Determine the acceleration of the skier at this instant. At this instant, the acceleration of the skier along with its direction is ? acceleration is 22.8 ft/s^2 but need help with direction. Need help with velocity direction and acceleration direction please.arrow_forwardFor Problems 18-22 (Table 7-27), design a V-belt drive. Specify the belt size, the sheave sizes, the number of belts, the actual output speed, and the center distance.arrow_forward
- only 21arrow_forwardonly 41arrow_forwardNormal and tangential components-relate to x-y coordinates A race car enters the circular portion of a track that has a radius of 65 m. When the car enters the curve at point P, it is traveling with a speed of 120 km/h that is increasing at 5 m/s^2 . Three seconds later, determine the x and y components of velocity and acceleration of the car. I need help with finding the y component of the total acceleration. I had put -32 but its incorrect. but i keep getting figures around that numberarrow_forward
- The bracket BCD is hinged at C and attached to a control cable at B. Let F₁ = 275 N and F2 = 275 N. F1 B a=0.18 m C A 0.4 m -0.4 m- 0.24 m Determine the reaction at C. The reaction at C N Z F2 Darrow_forwardConsider the angle bar shown in the given figure A W 240 mm B 80 mm Draw the free-body diagram needed to determine the reactions at A and B when a = 150 mm. This problem could also be approached as a 3-force body using method of Section 4.2B.arrow_forwardA telemetry system is used to quantify kinematic values of a ski jumper immediately before the jumper leaves the ramp. According to the system r=560 ft , r˙=−105 ft/s , r¨=−10 ft/s2 , θ=25° , θ˙=0.07 rad/s , θ¨=0.06 rad/s2 Determine the velocity of the skier immediately before leaving the jump. The velocity of the skier immediately before leaving the jump along with its direction is ? I have 112.08 ft/s but can't seem to get the direction correct. Determine the acceleration of the skier at this instant. At this instant, the acceleration of the skier along with its direction is ? acceleration is 22.8 ft/s^2 but need help with direction. Need help with velocity direction and acceleration direction please.arrow_forward
- For the stop bracket shown, locate the x coordinate of the center of gravity. Consider a = = 16.50 mm. 34 mm 62 mm 51 mm 10 mm 100 mm 88 mm 55 mm 45 mm The x coordinate of the center of gravity is mm.arrow_forwardIn the given figure, the bent rod ABEF is supported by bearings at C and D and by wire AH. The portion AB of the rod is 250 mm long, and the load W is 580 N. Assume that the bearing at D does not exert any axial thrust. H B A с 30° 250 mm D Z 50 mm 300 mm F 250 mm 50 mm W Draw the free-body diagram needed to determine the tension in wire AH and the reactions at C and D.arrow_forwardA 10-ft boom is acted upon by the 810-lb force as shown in the figure. D 6 ft 6 ft E B 7 ft C 6 ft x 4 ft W Draw the free-body diagram needed to determine the tension in each cable and the reaction at the ball-and-socket joint at A.arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





