
Organic Chemistry
12th Edition
ISBN: 9781118875766
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10, Problem 20P
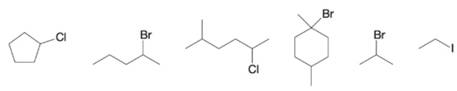
Which of the following compounds can be prepared by radical halogenations which little complication by formation of isomeric by-products?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw the formula of the product obtained by reacting adipic acid 1st with PCl5 and 2nd treatment with NH3.
please help me with my homework
help
Chapter 10 Solutions
Organic Chemistry
Ch. 10 - Prob. 1PPCh. 10 - Prob. 2PPCh. 10 - Practice Problem 10.3 How would the molecular ion...Ch. 10 - Prob. 4PPCh. 10 - Prob. 5PPCh. 10 - Prob. 6PPCh. 10 - Practice Problem 10.7 Chlorination reactions of...Ch. 10 - Prob. 8PPCh. 10 - Prob. 9PPCh. 10 - Prob. 10PP
Ch. 10 - Prob. 11PPCh. 10 - Practice Problem 10.12 Benzylic radicals, due to...Ch. 10 - Prob. 13PPCh. 10 - Practice Problem 10.14 Show how the following...Ch. 10 - Prob. 15PPCh. 10 - Prob. 16PPCh. 10 - Prob. 17PPCh. 10 - Prob. 18PCh. 10 - Explain the relative distribution of produces...Ch. 10 - 10.20 Which of the following compounds can be...Ch. 10 - Prob. 21PCh. 10 - Prob. 22PCh. 10 - Prob. 23PCh. 10 - Prob. 24PCh. 10 - 10.25 List in order of decreasing stability all of...Ch. 10 - Prob. 26PCh. 10 - Prob. 27PCh. 10 - Prob. 28PCh. 10 - Starting with the compound or compounds indicated...Ch. 10 - Prob. 30PCh. 10 - 10.31 Synthesize each of the following compounds...Ch. 10 - Synthesize each of die following compounds by...Ch. 10 - Prob. 33PCh. 10 - Prob. 34PCh. 10 - Prob. 36PCh. 10 - The halogen atom of an alkyl halide can be...Ch. 10 - Prob. 38PCh. 10 - Prob. 39PCh. 10 - Write a mechanism for the following reaction.Ch. 10 - 10.41 Hydrogen peroxide and ferrous sulfate react...Ch. 10 - Prob. 42PCh. 10 - If one were to try to draw the simplest Lewis...Ch. 10 - Prob. 1LGPCh. 10 - 2. (a) Propose a synthesis of 2-methoxypropene...
Additional Science Textbook Solutions
Find more solutions based on key concepts
47. A 250.0-mL buffer solution is 0.250 M in acetic acid and 0.250 M in sodium acetate.
a. What is the initial ...
Chemistry: A Molecular Approach (4th Edition)
The bioremediation process shown in the photograph is used to remove benzene and other hydrocarbons from soil c...
Microbiology: An Introduction
Examine the following diagrams of cells from an organism with diploid number 2n = 6, and identify what stage of...
Genetic Analysis: An Integrated Approach (3rd Edition)
SCIENTIFIC INQUIRY You are handed a mystery pea plant with tall stems and axial flowers and asked to determine ...
Campbell Biology (11th Edition)
8. A classic way to isolate thymidylate synthase—negative mutants of bacteria is to treat a growing culture wit...
Biochemistry: Concepts and Connections (2nd Edition)
The similarity and difference in ionic and covalent bond should be determined. Concept Introduction: The intera...
Living By Chemistry: First Edition Textbook
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forward
- er your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward5.arrow_forward6.arrow_forward
- 0/5 alekscgi/x/sl.exe/1o_u-IgNglkr7j8P3jH-IQs_pBaHhvlTCeeBZbufuBYTi0Hz7m7D3ZcSLEFovsXaorzoFtUs | AbtAURtkqzol 1HRAS286, O States of Matter Sketching a described thermodynamic change on a phase diagram The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 3 pressure (atm) + 0- 0 5+ 200 temperature (K) 400 Explanation Check X 0+ F3 F4 F5 F6 F7 S 2025 McGraw Hill LLC All Rights Reserved. Terms of Use Privacy Center Accessibility Q Search LUCR + F8 F9 F10 F11 F12 * % & ( 5 6 7 8 9 Y'S Dele Insert PrtSc + Backsarrow_forward5.arrow_forward9arrow_forward
- alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IQs_pBanHhvlTCeeBZbufu BYTI0Hz7m7D3ZS18w-nDB10538ZsAtmorZoFusYj2Xu9b78gZo- O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 3- 200 temperature (K) Explanation Chick Q Sowncharrow_forward0+ aleksog/x/lsl.exe/1ou-lgNgkr7j8P3H-IQs pBaHhviTCeeBZbufuBYTOHz7m7D3ZStEPTBSB3u9bsp3Da pl19qomOXLhvWbH9wmXW5zm O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 Gab The temperature on a sample of pure X held at 0.75 atm and -229. °C is increased until the sample sublimes. The temperature is then held constant and the pressure is decreased by 0.50 atm. On the phase diagram below draw a path that shows this set of changes. F3 pressure (atm) 0- 0 200 Explanation temperature (K) Check F4 F5 ☀+ Q Search Chill Will an 9 ENG F6 F7 F8 F9 8 Delete F10 F11 F12 Insert PrtSc 114 d Ararrow_forwardx + LEKS: Using a phase diagram a X n/alekscgi/x/lsl.exe/10_u-IgNsikr7j8P3jH-IQs_pBan HhvlTCeeBZbufu BYTI0Hz7m7D3ZcHYUt80XL-5alyVpw ○ States of Matter Using a phase diagram to find a phase transition temperature or pressure Use the phase diagram of Substance X below to find the melting point of X when the pressure above the solid is 1.1 atm. pressure (atm) 16 08- solid liquid- 0 200 400 gas 600 temperature (K) Note: your answer must be within 25 °C of the exact answer to be graded correct. × 5arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Characteristic Reactions of Benzene and Phenols; Author: Linda Hanson;https://www.youtube.com/watch?v=tjEqEjDd87E;License: Standard YouTube License, CC-BY
An Overview of Aldehydes and Ketones: Crash Course Organic Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=-fBPX-4kFlw;License: Standard Youtube License