
Organic Chemistry-Package(Custom)
4th Edition
ISBN: 9781259141089
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10, Problem 10.74P
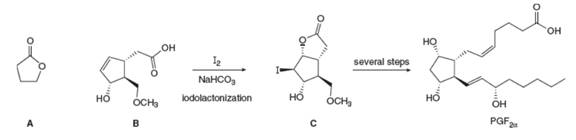
Lactones, cyclic esters such as compound A, are prepared by halolactonization, an addition reaction to an
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
3. Put the following species in order of increasing bond length by using molecular orbital diagrams and
calculating their bond orders: F2, F2, F2+
Molecular Orbital Diagram
F2
F2
F2+
Bond Order
Shortest bond:
Longest bond
3. Put the following species in order of increasing bond length by using molecular orbital diagrams and
calculating their bond orders: F2, F2, F2+
Molecular Orbital Diagram
F2
F2
F2+
Bond Order
4. The superoxide ion, Oz, plays an important role in the ageing processes that take place in organisms.
Judge whether Oz is likely to have larger or smaller dissociation energy than 02.
Molecular Orbital Diagram
02
02
Does O2 have larger or smaller dissociation energy?:
Bond Order
Chapter 10 Solutions
Organic Chemistry-Package(Custom)
Ch. 10 - Prob. 10.1PCh. 10 - Problem 10.2 How many degrees of unsaturation are...Ch. 10 - Give an example of a compound with molecular...Ch. 10 - Give the IUPAC name for each alkene.Ch. 10 - Give the IUPAC name for each polyfunctional...Ch. 10 - Prob. 10.6PCh. 10 - Prob. 10.7PCh. 10 - Prob. 10.8PCh. 10 - Prob. 10.9PCh. 10 - Problem 10.10 Rank the following isomers in order...
Ch. 10 - Linolenic acidTable 10.2 and stearidonic acid are...Ch. 10 - Prob. 10.12PCh. 10 - Prob. 10.13PCh. 10 - Prob. 10.14PCh. 10 - Prob. 10.15PCh. 10 - Prob. 10.16PCh. 10 - Prob. 10.17PCh. 10 - Prob. 10.18PCh. 10 - Prob. 10.19PCh. 10 - Which compounds A-D in Figure 10.12 are formed by...Ch. 10 - What two alkenes give rise to each alcohol as the...Ch. 10 - Prob. 10.22PCh. 10 - Problem 10.23 Draw the products of each reaction,...Ch. 10 - Problem 10.24 Draw all stereoisomers formed in...Ch. 10 - Prob. 10.25PCh. 10 - Prob. 10.26PCh. 10 - Borane is sold for laboratory use as a complex...Ch. 10 - What alkylborane is formed from hydroboration of...Ch. 10 - Draw the products formed when each alkene is...Ch. 10 - What alkene can be used to prepare each alcohol as...Ch. 10 - Prob. 10.31PCh. 10 - Draw the products of each reaction using the two...Ch. 10 - Problem 10.31 Devise a synthesis of each compound...Ch. 10 - Give the IUPAC name for each compound. a.b.Ch. 10 - a Label the carbon-carbon double bond in A as E or...Ch. 10 - Prob. 10.36PCh. 10 - Calculate the number of degrees of unsaturation f...Ch. 10 - Prob. 10.38PCh. 10 - The fertility drug clomiphene trade name Clomid is...Ch. 10 - Give the IUPAC name for each compound. a....Ch. 10 - Give the structure corresponding to each name. a....Ch. 10 - 10.40 (a) Draw all possible stereoisomers of, and...Ch. 10 - Prob. 10.43PCh. 10 - 10.42 Now that you have learned how to name...Ch. 10 - Prob. 10.45PCh. 10 - Prob. 10.46PCh. 10 - Prob. 10.47PCh. 10 - Prob. 10.48PCh. 10 - By using the bond dissociation energies in...Ch. 10 - Prob. 10.50PCh. 10 - Repeat Problem 10.50 with CH32C=CH2 as the...Ch. 10 - What alkene can be used to prepare each alkyl...Ch. 10 - Prob. 10.53PCh. 10 - Draw the constitutional isomer formed in each...Ch. 10 - Prob. 10.55PCh. 10 - Draw all stereoisomers formed in each reaction....Ch. 10 - Prob. 10.57PCh. 10 - Prob. 10.58PCh. 10 - Prob. 10.59PCh. 10 - Draw a stepwise mechanism for the following...Ch. 10 - Draw a stepwise mechanism for each reaction. a.b.Ch. 10 - Draw a stepwise mechanism that shows how all three...Ch. 10 - Less stable alkenes can be isomerized to more...Ch. 10 - Prob. 10.64PCh. 10 - Prob. 10.65PCh. 10 - Bromoetherification, the addition of the elements...Ch. 10 - Devise a synthesis of each product from the given...Ch. 10 - Prob. 10.68PCh. 10 - Prob. 10.69PCh. 10 - Prob. 10.70PCh. 10 - 10.66 Explain why A is a stable compound but B is...Ch. 10 - Prob. 10.72PCh. 10 - Prob. 10.73PCh. 10 - 10.69 Lactones, cyclic esters such as compound A,...Ch. 10 - 10.70 Draw a stepwise mechanism for the following...Ch. 10 - 10.71 Like other electrophiles, carbocations add...Ch. 10 - 10.72 Draw a stepwise mechanism for the...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Describe Mendels conclusions about how traits are passed from generation to generation.
Concepts of Genetics (12th Edition)
What process causes the Mediterranean intermediate Water MIW to become more dense than water in the adjacent At...
Applications and Investigations in Earth Science (9th Edition)
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
6. How can you use the features found in each chapter?
Human Anatomy & Physiology (2nd Edition)
56. Global Positioning System. Learn more about the global positioning system and its uses. Write a short repo...
The Cosmic Perspective (8th Edition)
An obese 55-year-old woman consults her physician about minor chest pains during exercise. Explain the physicia...
Biology: Life on Earth with Physiology (11th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- On what basis are Na and Nb ranked against each other?arrow_forwardStep 1: add a curved arrow. Select Draw Templates More / " C H Br 0 Br : :o: Erase H H H H Q2Q Step 2: Draw the intermediates and a curved arrow. Select Draw Templates More MacBook Air / " C H Br 0 9 Q Erase 2Qarrow_forwardO Macmillan Learning Question 23 of 26 > Stacked Step 7: Check your work. Does your synthesis strategy give a substitution reaction with the expected regiochemistry and stereochemistry? Draw the expected product of the forward reaction. - - CN DMF MacBook Air Clearly show stereochemistry. Questionarrow_forward
- NH2 1. CH3–MgCl 2. H3O+ ? As the lead product manager at OrganometALEKS Industries, you are trying to decide if the following reaction will make a molecule with a new C - C bond as its major product: If this reaction will work, draw the major organic product or products you would expect in the drawing area below. If there's more than one major product, you can draw them in any arrangement you like. Be sure you use wedge and dash bonds if necessary, for example to distinguish between major products with different stereochemistry. If the major products of this reaction won't have a new C - C bond, just check the box under the drawing area and leave it blank. Click and drag to start drawing a structure. This reaction will not make a product with a new C - C bond. Х ☐: Carrow_forwardPredict the major products of this organic reaction. If there will be no major products, check the box under the drawing area instead. No reaction. : + Х è OH K Cr O 2 27 2 4' 2 Click and drag to start drawing a structure.arrow_forwardLaminar compounds are characterized by havinga) a high value of the internal surface of the solid.b) a high adsorption potential.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
CBSE Class 12 Chemistry || Polymers || Full Chapter || By Shiksha House; Author: Best for NEET;https://www.youtube.com/watch?v=OxdJlS0xZ0Y;License: Standard YouTube License, CC-BY