
Concept explainers
Draw the constitutional isomer formed in each reaction.
a. ![]() d.
d.![]() g.
g.![]()
b.![]() e.
e.![]() h.
h.![]()
c.![]() f.
f. ![]()
(a)
Interpretation: The constitutional isomer formed in the given reaction is to be drawn.
Concept introduction: A reaction of hydrogen halide with alkene results in the formation of alkyl halide. This type of reaction is an electrophilic addition of hydrogen halide. Electrophilic addition reaction follows markovnikov�s rule.
According to markovnikov�s rule, the positive part of halogen acid attached to that carbon atom in
Answer to Problem 10.54P
The constitutional isomer formed in the given reaction is shown below:
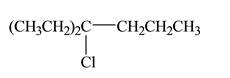
Explanation of Solution
A reaction of hydrogen halide with alkene results in the formation of alkyl halide. This type of reaction is an electrophilic addition of hydrogen halide.
The steps followed by electrophilic addition reaction are stated below:
• First protonation of the alkene take place to generate the carbocation.
• The halide ion will attack on the carbocation to give the final product
The constitutional isomer formed in the given reaction is shown below.
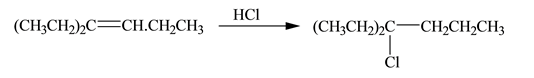
Figure 1
Therefore, the constitutional isomer formed in the given reaction is
The constitutional isomer formed in the given reaction is
(b)
Interpretation: The constitutional isomer formed in the given reaction is to be drawn.
Concept introduction: Hydration of alkenes is one the method used for the formation of alcohol.
The general steps followed by hydration reaction are stated below:
• First protonation of the alkene take place to generate the carbocation.
• Formation of protonated alcohol.
• Deprotonation.
Answer to Problem 10.54P
The constitutional isomer formed in the given reaction is
Explanation of Solution
Hydration of alkenes is one the method used for the formation of alcohol.
The general steps followed by hydration reaction are stated below:
• First protonation of the alkene take place to generate the carbocation.
• Formation of protonated alcohol.
• Deprotonation.
In this type of reaction, hydroxyl group attacks on the more substituted carbon.
The constitutional isomer formed in the given reaction is shown below.
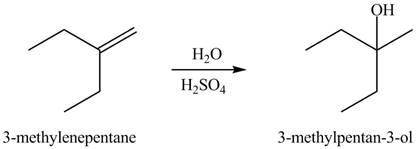
Figure 2
The first step in the given reaction is the abstraction of proton from sulfuric acid. This results in the formation of carbocation. In the next step, attack of water molecule takes place on the electrophilic carbon. The last step is the removal of
The constitutional isomer formed in the given reaction is
(c)
Interpretation: The constitutional isomer formed in the given reaction is to be drawn.
Concept introduction: Hydroboration reaction is a two step reaction, which involves conversion of alkene into alcohol. This type of reaction follows anti-markovnikov�s rule.
Anti markovnikov�s rule states that the positive part of acid attached to that carbon atom in
Answer to Problem 10.54P
The constitutional isomer formed in the given reaction is
Explanation of Solution
Hydroboration reaction is a two step reaction, which involves conversion of alkene into alcohol. This type of reaction follows anti-markovnikov�s rule.
Anti markovnikov�s rule states that the positive part of acid attached to that carbon atom in
The constitutional isomer formed in the given reaction is shown below.
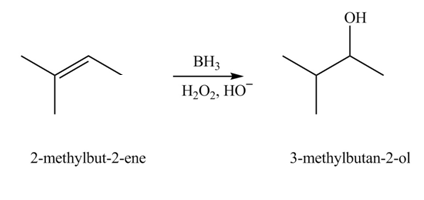
Figure 3
During hydroboration of
Therefore, the product formed is
The constitutional isomer formed in the given reaction is
(d)
Interpretation: The constitutional isomer formed in the given reaction is to be drawn.
Concept introduction: The addition of a halogen to an alkyne chain leads to the formation of corresponding alkene or alkane. The reaction which includes the addition of chlorine atoms to the alkyne chain is known as chlorination. Chlorination can be done by using the reagents like
Answer to Problem 10.54P
The constitutional isomer formed in the given reaction is
Explanation of Solution
Chlorine gas is added to the double bond of the cyclohexene. Addition of chlorine is known as chlorination and this addition makes the compound saturated.
The constitutional isomer formed in the given reaction is shown below.
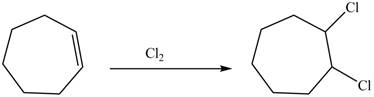
Figure 4
Therefore, the product formed is
The constitutional isomer formed in the given reaction is
(e)
Interpretation: The constitutional isomer formed in the given reaction is to be drawn.
Concept introduction: The reaction of alkene with halogen and water results in the formation of halohydrin product.
The general steps for the formation of halohydrin are stated below:
• The first step is the attack of halide ion to form a halonium ion.
• The second step is the attack of water from back side to opens the halonium ion.
• The last step is deprotonation to give the halohydrin product.
Answer to Problem 10.54P
The constitutional isomer formed in the given reaction is
Explanation of Solution
The reaction of alkene with halogen and water results in the formation of halohydrin product.
The general steps for the formation of halohydrin are stated below:
• The first step is the attack of halide ion to form a halonium ion.
• The second step is the attack of water from back side to opens the halonium ion.
• The last step is deprotonation to give the halohydrin product.
The constitutional isomer formed in the given reaction is shown below.
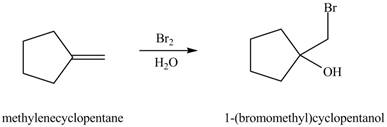
Figure 5
Therefore, the product formed is
The constitutional isomer formed in the given reaction is
(f)
Interpretation: The constitutional isomer formed in the given reaction is to be drawn.
Concept introduction: Hydroboration reaction is a two step reaction, which involves conversion of alkene into alcohol. This type of reaction follows anti-markovnikov�s rule.
Anti markovnikov�s rule states that the positive part of acid attached to that carbon atom in
Answer to Problem 10.54P
The constitutional isomer formed in the given reaction is cyclohexylmethanol.
Explanation of Solution
Hydroboration reaction is a two step reaction, which involves conversion of alkene into alcohol. This type of reaction follows anti-markovnikov�s rule.
Anti Markovnikov’s rule states that the positive part of acid attached to that carbon atom in
The constitutional isomer formed in the given reaction is shown below.
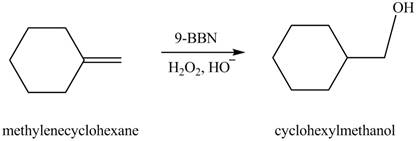
Figure 6
Therefore, the product formed is cyclohexylmethanol.
The constitutional isomer formed in the given reaction is cyclohexylmethanol.
(g)
Interpretation: The constitutional isomer formed in the given reaction is to be drawn.
Concept introduction: The free radical bromination of alkenes takes place at allylic position. NBS generates bromine radical in presence of heat or light. More than one product is formed when the allylic radical is resonance stabilized.
Answer to Problem 10.54P
The constitutional isomer formed in the given reaction is
Explanation of Solution
The general steps for the formation of halohydrin are stated below:
• The first step is the attack of halide ion to form a halonium ion.
• The second step is the attack of water from back side to opens the halonium ion.
• The last step is deprotonation to give the halohydrin product.
The constitutional isomer formed in the given reaction is shown below.
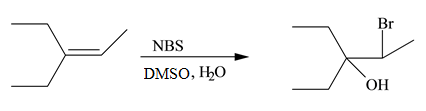
Figure 7
Therefore, the product formed is
The constitutional isomer formed in the given reaction is
(h)
Interpretation: The constitutional isomer formed in the given reaction is to be drawn.
Concept introduction: The general steps followed by the addition of Halogens to alkenes are stated below:
• The first step is the electrophilic attack of halide ion to form a halonium ion.
• The second step is the attack of halide ion from back side to opens the halonium ion.
Answer to Problem 10.54P
The constitutional isomer formed in the given reaction is
Explanation of Solution
The general steps involved in the addition of Halogens to alkenes are stated below:
• The first step is the electrophilic attack of halide ion to form a halonium ion.
• The second step is the attack of halide ion from back side to opens the halonium ion to give the final product.
The constitutional isomer formed in the given reaction is shown below.
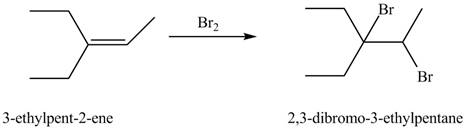
Figure 8
Therefore, the constitutional isomer formed in the given reaction is
The constitutional isomer formed in the given reaction is
Want to see more full solutions like this?
Chapter 10 Solutions
Organic Chemistry-Package(Custom)
- help 20arrow_forwardProvide the drawing of the unknown structure that corresponds with this data.arrow_forward20.44 The Diels-Alder reaction is not limited to making six-membered rings with only car- bon atoms. Predict the products of the following reactions that produce rings with atoms other than carbon in them. OCCH OCCH H (b) CH C(CH₂)s COOCH མ་ནས་བ (c) N=C H -0.X- (e) H C=N COOCHS + CH2=CHCH₂ →→arrow_forward
- 3) Draw a detailed mechanism and predict the product of the reaction shown? 1) EtMgBr 2) H3O+arrow_forwardHow to draw the mechanism for this reaction?arrow_forward> H₂C=C-CH2-CH3 B. H₂O Pt C. + H2 + H₂O H D. 16. Give the IUPAC name for each of the following: B. Cl Cl c. Cl Cl 17. Draw the line-angle formula for each of the following compounds: 1. phenol 2. 1,3-dichlorobenzene 3. 4-ethyltoluene < Previous Submit Assignment Next ▸arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





