
Organic Chemistry-Package(Custom)
4th Edition
ISBN: 9781259141089
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10, Problem 10.52P
What
addition reaction?
a.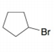 b.
b. 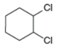 c.
c. 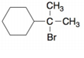 d.
d. 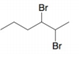 e.
e. 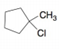 f.
f.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Calculating the pH at equivalence of a titration
Try Again
Your answer is incorrect.
0/5
a
A chemist titrates 70.0 mL of a 0.7089 M hydrocyanic acid (HCN) solution with 0.4574M KOH solution at 25 °C. Calculate the pH at equivalence. The pK of
hydrocyanic acid is 9.21.
Round your answer to 2 decimal places.
Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of KOH solution added.
pH
=
11.43]
G
00.
18
Ar
B•
Biological Macromolecules
Naming and drawing the products of aldose oxidation and reduction
aw a Fischer projection of the molecule that would produce L-ribonic acid if it were subjected to mildly oxidizing reaction conditions.
Click and drag to start drawing a
structure.
X
AP
‡
1/5
Naor
Explanation
Check
McGraw Hill LLC. All Rights Reserved. Terms of Use
Privacy Center
Accessibil
● Biological Macromolecules
Identifying the parts of a disaccharide
Take a look at this molecule, and then answer the questions in the table below it.
CH2OH
O
H
H
H
OH
OH
OH
H
H
CH2OH
H
O
OH
H
OH H
H
H
H
OH
Is this a reducing sugar?
Does this molecule contain a glycosidic bond?
If you said this molecule does contain a glycosidic bond, write the symbol
describing it.
If you said this molecule does contain a glycosidic bond, write the common
names (including anomer and enantiomer labels) of the molecules that
would be released if that bond were hydrolyzed.
If there's more than one molecule, separate each name with a comma.
Explanation
Check
O yes
X
O no
○ yes
O no
U
Chapter 10 Solutions
Organic Chemistry-Package(Custom)
Ch. 10 - Prob. 10.1PCh. 10 - Problem 10.2 How many degrees of unsaturation are...Ch. 10 - Give an example of a compound with molecular...Ch. 10 - Give the IUPAC name for each alkene.Ch. 10 - Give the IUPAC name for each polyfunctional...Ch. 10 - Prob. 10.6PCh. 10 - Prob. 10.7PCh. 10 - Prob. 10.8PCh. 10 - Prob. 10.9PCh. 10 - Problem 10.10 Rank the following isomers in order...
Ch. 10 - Linolenic acidTable 10.2 and stearidonic acid are...Ch. 10 - Prob. 10.12PCh. 10 - Prob. 10.13PCh. 10 - Prob. 10.14PCh. 10 - Prob. 10.15PCh. 10 - Prob. 10.16PCh. 10 - Prob. 10.17PCh. 10 - Prob. 10.18PCh. 10 - Prob. 10.19PCh. 10 - Which compounds A-D in Figure 10.12 are formed by...Ch. 10 - What two alkenes give rise to each alcohol as the...Ch. 10 - Prob. 10.22PCh. 10 - Problem 10.23 Draw the products of each reaction,...Ch. 10 - Problem 10.24 Draw all stereoisomers formed in...Ch. 10 - Prob. 10.25PCh. 10 - Prob. 10.26PCh. 10 - Borane is sold for laboratory use as a complex...Ch. 10 - What alkylborane is formed from hydroboration of...Ch. 10 - Draw the products formed when each alkene is...Ch. 10 - What alkene can be used to prepare each alcohol as...Ch. 10 - Prob. 10.31PCh. 10 - Draw the products of each reaction using the two...Ch. 10 - Problem 10.31 Devise a synthesis of each compound...Ch. 10 - Give the IUPAC name for each compound. a.b.Ch. 10 - a Label the carbon-carbon double bond in A as E or...Ch. 10 - Prob. 10.36PCh. 10 - Calculate the number of degrees of unsaturation f...Ch. 10 - Prob. 10.38PCh. 10 - The fertility drug clomiphene trade name Clomid is...Ch. 10 - Give the IUPAC name for each compound. a....Ch. 10 - Give the structure corresponding to each name. a....Ch. 10 - 10.40 (a) Draw all possible stereoisomers of, and...Ch. 10 - Prob. 10.43PCh. 10 - 10.42 Now that you have learned how to name...Ch. 10 - Prob. 10.45PCh. 10 - Prob. 10.46PCh. 10 - Prob. 10.47PCh. 10 - Prob. 10.48PCh. 10 - By using the bond dissociation energies in...Ch. 10 - Prob. 10.50PCh. 10 - Repeat Problem 10.50 with CH32C=CH2 as the...Ch. 10 - What alkene can be used to prepare each alkyl...Ch. 10 - Prob. 10.53PCh. 10 - Draw the constitutional isomer formed in each...Ch. 10 - Prob. 10.55PCh. 10 - Draw all stereoisomers formed in each reaction....Ch. 10 - Prob. 10.57PCh. 10 - Prob. 10.58PCh. 10 - Prob. 10.59PCh. 10 - Draw a stepwise mechanism for the following...Ch. 10 - Draw a stepwise mechanism for each reaction. a.b.Ch. 10 - Draw a stepwise mechanism that shows how all three...Ch. 10 - Less stable alkenes can be isomerized to more...Ch. 10 - Prob. 10.64PCh. 10 - Prob. 10.65PCh. 10 - Bromoetherification, the addition of the elements...Ch. 10 - Devise a synthesis of each product from the given...Ch. 10 - Prob. 10.68PCh. 10 - Prob. 10.69PCh. 10 - Prob. 10.70PCh. 10 - 10.66 Explain why A is a stable compound but B is...Ch. 10 - Prob. 10.72PCh. 10 - Prob. 10.73PCh. 10 - 10.69 Lactones, cyclic esters such as compound A,...Ch. 10 - 10.70 Draw a stepwise mechanism for the following...Ch. 10 - 10.71 Like other electrophiles, carbocations add...Ch. 10 - 10.72 Draw a stepwise mechanism for the...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The aim of the lab is to measure the sodium content from tomato sauce using the Mohr titration method. There are two groups being: Regular Tomato sauce & Salt Reduced tomato sauce QUESTION: State how you would prepare both Regular & Salt reduced tomato sauce samples for chemical analysis using the Mohr titration methodarrow_forwardUsing the conditions of spontaneity to deduce the signs of AH and AS Use the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction observations conclusions A The reverse of this reaction is always spontaneous but proceeds faster at temperatures above -48. °C. ΔΗ is (pick one) ✓ AS is (pick one) B This reaction is spontaneous except below 114. °C but proceeds at a slower rate below 135. °C. ΔΗ is (pick one) AS is (pick one) ΔΗ is C This reaction is exothermic and proceeds faster at temperatures above -43. °C. (pick one) AS is (pick one) v Х 5 ? 18 Ararrow_forwardion. A student proposes the following Lewis structure for the perchlorate (CIO) io : :0: : Cl : - - : :0: ك Assign a formal charge to each atom in the student's Lewis structure. atom central O formal charge ☐ top O ☐ right O ☐ bottom O ☐ Cl ☐arrow_forward
- Decide whether these proposed Lewis structures are reasonable. proposed Lewis structure Yes. Is the proposed Lewis structure reasonable? Cl- : 2: :Z: :Z: N—N : 0: C C1: O CO No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* ☐ Yes. No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* | Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* | If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many times as necessary. For example, if two oxygen atoms don't satisfy the octet rule, enter "0,0". ☑arrow_forwardUse the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction observations conclusions ΔΗ is (pick one) A This reaction is faster above 103. °C than below. AS is (pick one) ΔΗ is (pick one) B This reaction is spontaneous only above -9. °C. AS is (pick one) ΔΗ is (pick one) C The reverse of this reaction is always spontaneous. AS is (pick one) 18 Ararrow_forwardUse the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction observations conclusions A The reverse of this reaction is always spontaneous but proceeds slower at temperatures below 41. °C. ΔΗ is (pick one) AS is (pick one) ΔΗ is (pick one) B This reaction is spontaneous except above 94. °C. AS is (pick one) This reaction is always spontaneous, but ΔΗ is (pick one) C proceeds slower at temperatures below −14. °C. AS is (pick one) Х 00. 18 Ar 무ㅎ B 1 1arrow_forward
- Draw the product of the reaction shown below. Ignore inorganic byproducts. + H CH3CH2OH HCI Drawingarrow_forwardplease explain this in simple termsarrow_forwardK Most Reactive Na (3 pts) Can the metal activity series (shown on the right) or a standard reduction potential table explain why potassium metal can be prepared from the reaction of molten KCI and Na metal but sodium metal is not prepared from the reaction of molten NaCl and K metal? Show how (not). Ca Mg Al с Zn Fe Sn Pb H Cu Ag Au Least Reactivearrow_forward
- (2 pts) Why is O2 more stable as a diatomic molecule than S2?arrow_forwardDraw the Lewis structure for the polyatomic phosphite (PO¾³¯) a anion. Be sure to include all resonance structures that satisfy the octet rule. C I A [ ]¯arrow_forwardDecide whether these proposed Lewis structures are reasonable. proposed Lewis structure Is the proposed Lewis structure reasonable? Yes. :0: Cl C C1: 0=0: : 0 : : 0 : H C N No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* ☐ Yes. No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* Yes. ☐ No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* | * If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many times as necessary. For example, if two oxygen atoms don't satisfy the octet rule, enter "0,0".arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY