
Concept explainers
Repeat Problem 10.50 with (CH3)2C=CH2 as the starting material.
(a)
Interpretation: The products obtained when (CH3)2C=CH2 is treated with each of the following reagents is to be drawn.
Concept introduction: Hydrohalogenation is an electrophilic addition of hydrohalic acids (such as HCl, HBr) to alkenes to bestow the corresponding haloalkanes. Addition of hydrohalic acid to an unsymmetrical alkene follows Markovnikov's rule. According to this rule, ‘the acid hydrogen (H) gets bonded to the carbon with more hydrogen substituents and the halide (X) group gets bonded to the carbon with more alkyl substituents’. This phenomenon is due to the abstraction of a hydrogen atom by the alkene from the acid to for the most stable carbocation. The stability of carbocation follows this order: 3°>2°>1°.
Answer to Problem 10.51P
The reaction sequence is depicted as ;
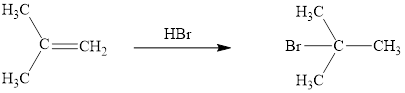
Explanation of Solution
The initial addition of proton to alkene 1 generates a tertiary carbocation by following Markovnikov's rule. Subsequently, bromide addition to the formed carbocation generates the product 2. Two bonds are cleaved in this reaction sequence i.e., the weak π-bond of the alkene and the HX bond of hydrohalic acid. Besides, two new sigma bonds are also formed such as one to H and one to X. The HBr bond polarized due to higher electronegativity of Br than H and the electrophilic H attracted to the electron rich double bond. Thus, these reactions are called as an ‘electrophilic additon’reactions.
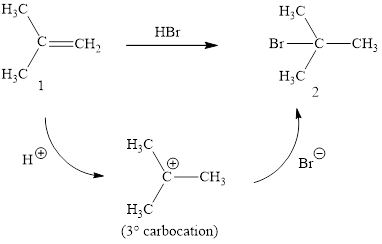
The HBr addition product of alkene is drawn.
(b)
Interpretation: The products obtained when (CH3)2C=CH2 is treated with each of the following reagents is to be drawn.
Concept introduction: Hydrohalogenation is an electrophilic addition of hydrohalic acids (such as HCl, HBr) to alkenes to bestow the corresponding haloalkanes. Addition of hydrohalic acid to a unsymmetrical alkene follows Markovnikov's rule. According to this rule, ‘the acid hydrogen (H) gets bonded to the carbon with more hydrogen substituents and the halide (X) group gets bonded to the carbon with more alkyl substituents’. This phenomenon is due to the abstraction of a hydrogen atom by the alkene from the acid to for the most stable carbocation. The stability of carbocation follows this order: 3°>2°>1°.
Answer to Problem 10.51P
The reaction sequence is depicted as ;
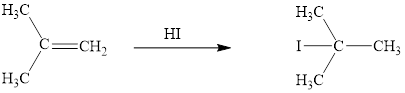
Explanation of Solution
The initial addition of proton to alkene 1 generates a tertiary carbocation by following Markovnikov's rule. Subsequently, iodide addition to the formed carbocation generates the product 2. Two bonds are cleaved in this reaction sequence i.e., the weak π-bond of the alkene and the HX bond of hydrohalic acid. Besides, two new sigma bonds are also formed such as one to H and one to X. The HI bond polarized due to higher electronegativity of I than H and the electrophilic H attracted to the electron rich double bond. Thus, these reactions are called as an ‘electrophilic additon’reactions.
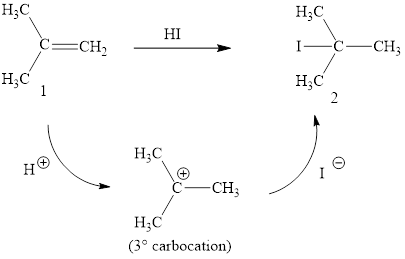
The HI addition product of alkene is drawn.
(c)
Interpretation: The products obtained when (CH3)2C=CH2 is treated with each of the following reagents is to be drawn.
Concept introduction: Hydration of alkene is an electrophilic addition of water in presence of acids to alkenes to bestow the corresponding hydration product. Addition of water to a unsymmetrical alkene follows Markovnikov's rule. According to this rule, ‘the acid hydrogen (H) gets bonded to the carbon with more hydrogen substituents and the OH group gets bonded to the carbon with more alkyl substituents’. This phenomenon is due to the abstraction of a hydrogen atom by the alkene from water to form the most stable carbocation. The stability of carbocation follows this order: 3°>2°>1°.
Answer to Problem 10.51P
The reaction sequence is depicted as ;
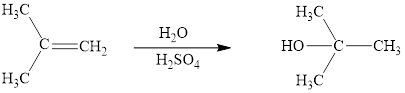
Explanation of Solution
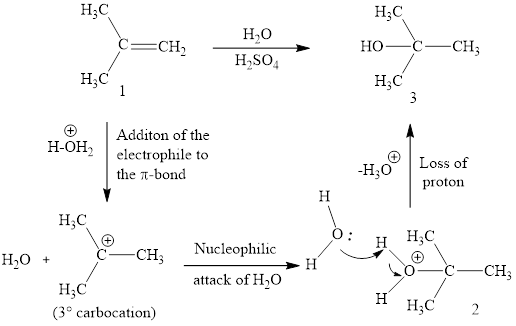
The initial addition of proton to alkene 1 generates a tertiary carbocation by following Markovnikov's rule. Subsequently, water addition to the formed carbocation generates the product 2. In further, the loss of proton furnishes the product alcohol 3. Two bonds are cleaved in this reaction sequence i.e., the weak π-bond of the alkene and the H2O bond of water. Besides, two new sigma bonds are also formed such as one to H and one to OH. Thus, these reactions are called as an ‘electrophilic additon’reactions.
The H2O addition product of alkene is drawn.
(d)
Interpretation: The products obtained when (CH3)2C=CH2 is treated with each of the following reagents is to be drawn.
Concept introduction: addition of alcohol to alkene is an electrophilic addition in presence of acids to alkenes to bestow the corresponding ether product. Addition of ethanol to a unsymmetrical alkene follows Markovnikov's rule. According to this rule, ‘the acid hydrogen (H) gets bonded to the carbon with more hydrogen substituents and the OEt group gets bonded to the carbon with more alkyl substituents’. This phenomenon is due to the abstraction of a hydrogen atom by the alkene from ethanol to form the most stable carbocation. The stability of carbocation follows this order: 3°>2°>1°.
Answer to Problem 10.51P
The reaction sequence is depicted as ;
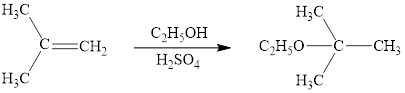
Explanation of Solution
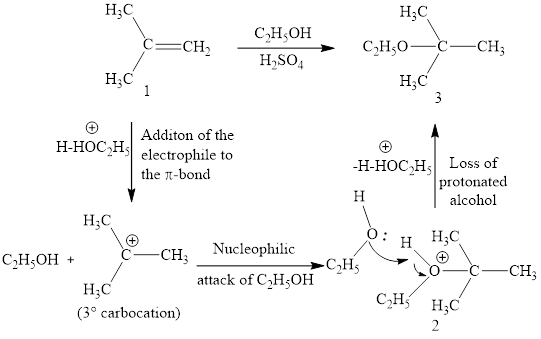
The initial addition of proton to alkene 1 generates a tertiary carbocation by following Markovnikov's rule. Subsequently, ethanol addition to the formed carbocation generates the product 2. In further, the loss of proton furnishes the product alcohol 3. Two bonds are cleaved in this reaction sequence i.e., the weak π-bond of the alkene and the -OH bond of ethanol. Besides, two new sigma bonds are also formed such as one to H and one to OC2H5. Thus, these reactions are called as an ‘electrophilic additon’reactions.
The ethanol addition product of alkene is drawn.
(e)
Interpretation: The products obtained when (CH3)2C=CH2 is treated with each of the following reagents is to be drawn.
Concept introduction: Halogenation reaction of alkene with Cl2 and Br2 is of high synthetic values. The electron rich alkene stimulates a dipole in halogen molecule, to make one halogen atom is electron deficient than the other one. This is followed by the addition of electrophilic halogen atom to nucleophilic alkene double bond.
Answer to Problem 10.51P
The reaction sequence is depicted as ;

Explanation of Solution
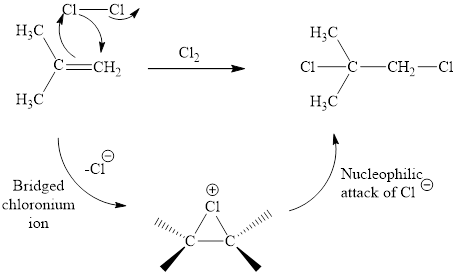
The initial addition of chlorine to alkene generates a bridged chloronium species. Subsequently, chloride addition to the formed bridged species generates the dichloro product. One double bond is cleaved in this reaction sequence i.e., the weak π-bond of the alkene and two new sigma bonds are also formed C-Cl. Thus, these reactions are called as an ‘electrophilic additon’reactions.
The Cl2 addition product of alkene is drawn.
(f)
Interpretation: The products obtained when (CH3)2C=CH2 is treated with each of the following reagents is to be drawn.
Concept introduction: Halohydrin reaction of alkene is an electrophilic addition in presence of water to bestow the corresponding hydroxy and halide substituted product. The electron rich alkene stimulates a dipole in halogen molecule, to make one halogen atom is electron deficient than the other one. This is followed by the addition of electrophilic halogen atom to nucleophilic alkene double bond.
Answer to Problem 10.51P
The reaction sequence is depicted as ;
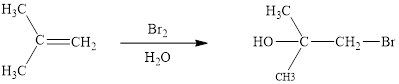
Explanation of Solution
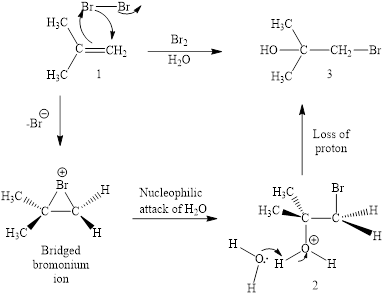
The initial addition of bromine to alkene 1 generates a bridged chloronium species. Subsequently, water addition to the formed bridged species generates the hydrated product 2.
Finally, the loss of proton results in the formation of bromohydrin product. One double bond is cleaved in this reaction sequence i.e., the weak π-bond of the alkene and two new sigma bonds are also formed C-Br and C-OH. Thus, these reactions are called as an ‘electrophilic additon’reactions.
The Br2/H2O addition product of alkene is drawn.
(g)
Interpretation: The products obtained when (CH3)2C=CH2 is treated with each of the following reagents is to be drawn.
Concept introduction: Halohydrin reaction of alkene is an electrophilic addition in presence of water to bestow the corresponding hydroxy and halide substituted product. The electron rich alkene stimulates a dipole in halogen molecule, to make one halogen atom is electron deficient than the other one. This is followed by the addition of electrophilic halogen atom to nucleophilic alkene double bond.
Answer to Problem 10.51P
The reaction sequence is depicted as ;
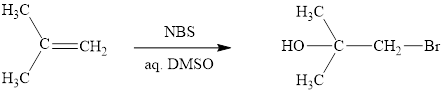
Explanation of Solution
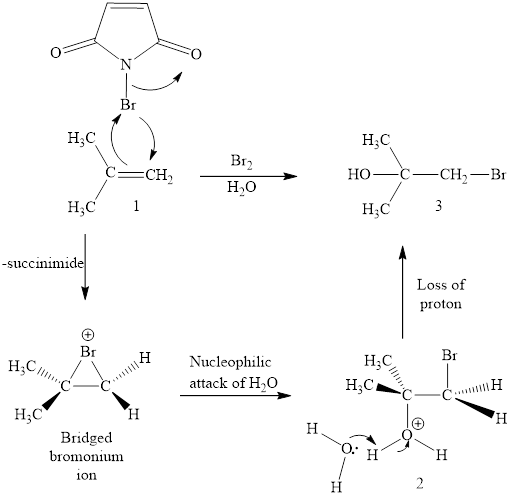
The initial addition of NBS to alkene 1 generates a bridged chloronium species with the expulsion of succinimide. Subsequently, water addition to the formed bridged species generates the hydrated product 2. Finally, the loss of proton results in the formation of bromohydrin product. One double bond is cleaved in this reaction sequence i.e., the weak π-bond of the alkene and two new sigma bonds are also formed C-Br and C-OH. Thus, these reactions are called as an ‘electrophilic additon’reactions.
The NBS in aq. DMSO addition product of alkene is drawn.
(h)
Interpretation: The products obtained when (CH3)2C=CH2 is treated with each of the following reagents is to be drawn.
Concept introduction: Hydroboration-oxidation is a two-step strategy to convert alkene to an alcohol. Borane generally exists as a dimer. Since, borane is a strong Lewis acid, it reacts with Lewis bases (ethers, amines, etc., )readily to form acid-base adducts. Addition of borane to alkene is a concerted reaction via syn addition.
Answer to Problem 10.51P
The reaction sequence is depicted as ;
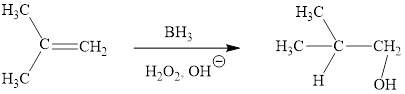
Explanation of Solution
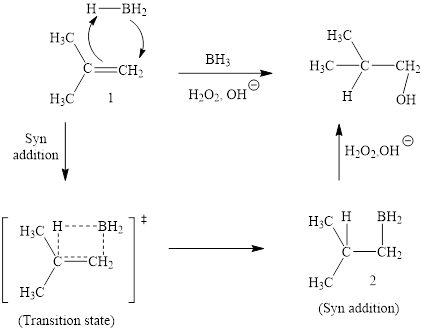
The reaction proceeds via concerted addition of H and BH2 from the same side of planar alkene 1 double bond. One π bond and one H-BH2 bond is cleaved with two new sigma bond formation 2. Since, four atoms are involved in the transition state, it is said to be ‘four centered’. Subsequently, alkaline oxidation with H2O2 results in the cleavage of C-BH2 bond to afford the alcohol. The overall addition of hydroboration-oxidation to alkene results in anti-markovnikov’s product.
The hydroboration-oxidation product of alkene is drawn.
(i)
Interpretation: The products obtained when (CH3)2C=CH2 is treated with each of the following reagents is to be drawn.
Concept introduction: Hydroboration-oxidation is a two-step strategy to convert alkene to an alcohol. Borane generally exists as a dimer. Since, borane is a strong Lewis acid, it reacts with Lewis bases (ethers, amines, etc., )readily to form acid-base adducts. Addition of borane to alkene is a concerted reaction via syn addition.
Answer to Problem 10.51P
The reaction sequence is depicted as ;
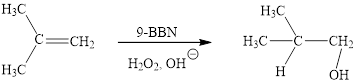
Explanation of Solution
The reaction proceeds via concerted addition of H and H-9BBN from the same side of planar alkene 1 double bond. One π bond and one H-9BBN bond is cleaved with two new sigma bond formation 2. Since, four atoms are involved in the transition state, it is said to be ‘four centered’. Subsequently, alkaline oxidation with H2O2 results in the cleavage of C-BH2 bond to afford the alcohol. The overall addition of hydroboration-oxidation to alkene results in anti-markovnikov’s product.
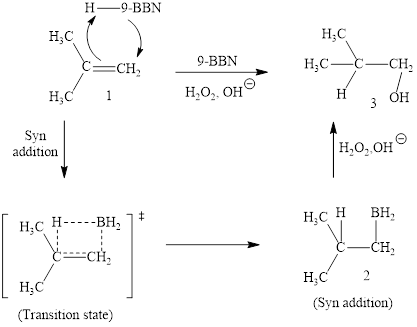
The hydroboration-oxidation product of alkene is drawn.
Want to see more full solutions like this?
Chapter 10 Solutions
Organic Chemistry-Package(Custom)
- Please correct answer and don't used hand raitingarrow_forwardhello, this is about physical chemistry . can you help me please?arrow_forwardPROBLEM 5+ What is the major product of each of the following reactions? a. CH3CH2CHCH3 + HBr d. + HBr A OH OH CH3 CH3 e. b. -OH + HCI + HCl A, OH CH3 OH CH3 c. CH3C CHCH3 + HBr CH3 OH f. CHCH3 + HCl ^>arrow_forward
- One suggestion for solving the fuel shortage due to decreasing volumes of fossil fuels are hydrogen / oxygen fuel cells. a. State the two half-cell reaction equations for such fuel cells. Calculate the cell potential as well as the electrical work gained by this fuel cell at standard conditions with E002/H20 = 1.229 V. b. Compare the fuel cell to the Gibbs free energy of the combustion reaction of n-octane at standard conditions. Use ASºm, n-Oct., 1 = 361.2 J/mol K.arrow_forwarda. Determine the electrochemical potential of the following cell using E°Mg2+/Mg = -2.362 V. Mg | Mg2+ (a=104) || H* (a = 4) | H2 (p = 0.5 bar) | Pt b. A galvanic chain consists of Co²+ / Co and Ag+ / Ag half-cells with EºCo²+/Co = -0.282 V and Eº Ag+/Ag = 0.799 V. Determine which half-cell will be reduced and which one will be oxidised. Furthermore, calculate the electrochemical potential as well as the equilibrium constant of the whole cell at i. [Co²+] = 0.1 M and [Ag+] = 0.5 M ii. [Co²+] = 0.001 M and [Ag*] = 1.5 Marrow_forwardThe equilibrium voltage of the following cell has been measured at 0.522 V at 25 °C. Pt | H2, g❘ HClaq || AgClaq | Ags State the redox reactions present in this cell. Calculate the pH value of the electrolyte solution with KL, AgCl = 1.96 · 10-10 mol² / L². Assume that the concentrations of H+ and Clare equal.arrow_forward
- Here are the energies (in kcal/mol) for staggered and eclipsed interactions for CH, CC, and CBr bonds eclipsed (0°) staggered (60°) bonds CH/CH 1.0 0.0 CH/CC 1.3 0.0 Br: CC/CC 3.0 0.9 Br CH/CBr 1.8 0.0 CC / CBr 3.3 1.0 CBr / CBr 3.7 1.2 a) I've drawn the Newman projection for one of the staggered conformations of the molecule above, looking down the C2-C3 bond. Draw Newman projections for the other two staggered and the three eclipsed conformations (in order). CH₂ H3C. H' H Br b) Calculate the relative energies for each of the conformations and write them below each conformation.arrow_forward90. Draw the stereoisomers obtained from each of the following reactions: a. H₂ b. H₂ C. H₂ Pd/C Pd/C Pd/Carrow_forward36. The emission spectrum below for a one-electron (hydrogen-like) species in the gas phase shows all the lines, before they merge together, resulting from transitions to the first excited state from higher energy states. Line A has a wavelength of 434 nm. BA Increasing wavelength, λ (a) What are the upper and lower principal quantum numbers corresponding to the lines labeled A and B? (b) Identify the one-electron species that exhibits the spectrum.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





