
Introduction to General, Organic and Biochemistry
11th Edition
ISBN: 9781285869759
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10, Problem 10.57P
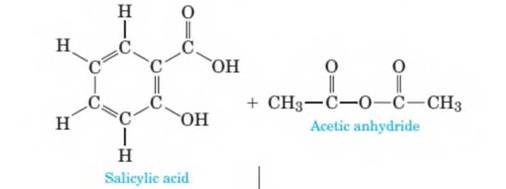
Aspirin is prepared by the reaction of salicylic- acid with acetic anhydride as shown in the following equation. The stoichiometry of the reaction is given in the equation. Acetic acid is a by-product of the reaction and must be separated and removed so that aspirin can then be sold as a pure product. How many grams of aspirin can be prepared from 120 grams of salicylic acid? Assume that there is an excess of acetic anhydride. (Chapter 4)
Acetylsalieylic acid
(Aspirin)
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
1.57 Draw all reasonable resonance structures for the following cation. Then draw the
resonance hybrid.
For the two questions below, draw the mechanism and form the major product.
Indicate similarities and differences between natural, exchanged and pillared clays.
Chapter 10 Solutions
Introduction to General, Organic and Biochemistry
Ch. 10.3 - Prob. 10.1PCh. 10.4 - Prob. 10.2PCh. 10.4 - Prob. 10.3PCh. 10.4 - Prob. 10.4PCh. 10.4 - Prob. 10.5PCh. 10.4 - Prob. 10.6PCh. 10 - Prob. 10.7PCh. 10 - Prob. 10.8PCh. 10 - 10-9 Is there any difference between vanillin made...Ch. 10 - Prob. 10.10P
Ch. 10 - 10-11 What important experiment did Wohler carry...Ch. 10 - Prob. 10.12PCh. 10 - Prob. 10.13PCh. 10 - Prob. 10.14PCh. 10 - 10-15 How many electrons are in the valence shell...Ch. 10 - 10-16 What is the relationship between the number...Ch. 10 - Prob. 10.17PCh. 10 - Prob. 10.18PCh. 10 - 10-19 Write Lewis structures for these ions. (a)...Ch. 10 - 10-20 Why are the following molecular formulas...Ch. 10 - 10-21 Explain how to use the valence-shell...Ch. 10 - 10-22 Suppose you forget to take into account the...Ch. 10 - Suppose you forget to take into account the...Ch. 10 - 10-24 Use the VSEPR model to predict the bond...Ch. 10 - Prob. 10.25PCh. 10 - Prob. 10.26PCh. 10 - 10-27 What is meant by the term functional group?Ch. 10 - 10-28 List three reasons why functional groups are...Ch. 10 - Prob. 10.29PCh. 10 - Prob. 10.30PCh. 10 - Prob. 10.31PCh. 10 - 10-32 Draw a structural formula for the one...Ch. 10 - 10-33 What is the meaning of the term tertiary (...Ch. 10 - Prob. 10.34PCh. 10 - Draw structural formulas for each of the...Ch. 10 - 10-36 Draw structural formulas for the six ketones...Ch. 10 - 10-37 Draw structural formulas for the eight...Ch. 10 - Prob. 10.38PCh. 10 - 10-39 (Chemical Connections 10A) How was Taxol...Ch. 10 - Prob. 10.40PCh. 10 - Prob. 10.41PCh. 10 - Silicon is immediately below carbon in Group 4A of...Ch. 10 - 10-43 Phosphorus is immediately below nitrogen in...Ch. 10 - Draw the structure for a compound with the...Ch. 10 - 10-45 Draw structural formulas for the eight...Ch. 10 - Prob. 10.46PCh. 10 - 10-47 Which of these covalent bonds are polar, and...Ch. 10 - Of the bonds in Problem 10-47, which is the most...Ch. 10 - Prob. 10.49PCh. 10 - Prob. 10.50PCh. 10 - Following is a structural formula for naphthalene....Ch. 10 - Prob. 10.52PCh. 10 - Prob. 10.53PCh. 10 - Urea, (NH.,)2CO, is used in plastics and in fertil...Ch. 10 - Prob. 10.55PCh. 10 - Prob. 10.56PCh. 10 - Aspirin is prepared by the reaction of salicylic-...Ch. 10 - Following is the structural formula of acetamide....Ch. 10 - Prob. 10.59P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Show work. don't give Ai generated solutionarrow_forwardIn intercalation compounds, their sheets can be neutral or have a negative or positive charge, depending on the nature of the incorporated species and its structure. Is this statement correct?arrow_forwardThis thermodynamic cycle describes the formation of an ionic compound MX2 from a metal element M and nonmetal element X in their standard states. What is the lattice enthalpy of MX2 ? What is the enthalpy formation of MX2 ? Suppose both the heat of sublimation of M and the ionization enthalpy of M were smaller. Would MX2 be more stable? Or less? or impossible to tell without more information?arrow_forward
- I need to make 25mL of solution with the stocks shown below. How would I calculate the math?arrow_forwardWe are practicing calculating for making solutions. How would I calculate this?arrow_forwardBr. , H+ .OH Mg ether solvent H+, H₂O 17. Which one of the compounds below is the final product of the reaction sequence shown above? HO A HO HO OH D B OH HO OH C OH HO OH Earrow_forward
- 8:57 PM Sun Jan 26 Content ← Explanation Page X Content X ALEKS Jade Nicol - Le A https://www-av C www-awa.aleks.com O States of Matter Understanding consequences of important physical properties of liquids ? QUESTION Liquid A is known to have a lower viscosity and lower surface tension than Liquid B. Use these facts to predict the result of each experiment in the table below, if you can. experiment Liquid A and Liquid B are each pumped through tubes with an inside diameter of 27.0 mm, and the pressures PA and PB needed to produce a steady flow of 2.4 mL/s are measured. 25.0 mL of Liquid A are poured into a beaker, and 25.0 mL of Liquid B are poured into an identical beaker. Stirrers in each beaker are connected to motors, and the forces FA and FB needed to stir each liquid at a constant rate are measured. predicted outcome OPA will be greater than PB OPA will be less than PB OPA will be equal to PB It's impossible to predict whether PA or PB will be greater without more information.…arrow_forwardShow work. Don't give Ai generated solutionarrow_forward5. Please draw in the blanks the missing transition states and the correlated products. Explicitly display relevant absolute stereochemical configuration. MeOH I OMe H Endo transition state, dienophile approaching from the bottom of diene + H ཎྞཾ ཌཱརཱ༔,_o OMe H H OMe Endo transition state, dienophile approaching from the top of diene or from the bottom but horizontally flipped (draw one) + Exo transition state, dienophile approaching from the top of diene or from the bottom but horizontally flipped (draw one) Exo transition state, dienophile approaching from the top of diene or from the bottom but horizontally flipped (draw one) MeO H H MeO H MeO H MeO H Harrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License