
Introduction to General, Organic and Biochemistry
11th Edition
ISBN: 9781285869759
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10, Problem 10.24P
10-24 Use the VSEPR model to predict the bond angles and geometry about each highlighted atom. (Hint-. Remember to take into account the presence of unshared pairs of electrons.) H H
I I
- H-C-C-O-H I I H H
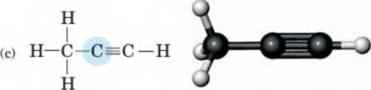
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Please help me calculate the undiluted samples ppm concentration.
My calculations were 280.11 ppm. Please see if I did my math correctly using the following standard curve.
Link: https://mnscu-my.sharepoint.com/:x:/g/personal/vi2163ss_go_minnstate_edu/EVSJL_W0qrxMkUjK2J3xMUEBHDu0UM1vPKQ-bc9HTcYXDQ?e=hVuPC4
Provide an IUPAC name for each of the compounds shown.
(Specify (E)/(Z) stereochemistry, if relevant, for straight chain alkenes only. Pay attention to
commas, dashes, etc.)
H₁₂C
C(CH3)3
C=C
H3C
CH3
CH3CH2CH
CI
CH3
Submit Answer
Retry Entire Group
2 more group attempts remaining
Previous
Next
Arrange the following compounds / ions in increasing nucleophilicity (least to
most nucleophilic)
CH3NH2
CH3C=C:
CH3COO
1
2
3
5
Multiple Choice 1 point
1, 2, 3
2, 1, 3
3, 1, 2
2, 3, 1
The other answers are not correct
0000
Chapter 10 Solutions
Introduction to General, Organic and Biochemistry
Ch. 10.3 - Prob. 10.1PCh. 10.4 - Prob. 10.2PCh. 10.4 - Prob. 10.3PCh. 10.4 - Prob. 10.4PCh. 10.4 - Prob. 10.5PCh. 10.4 - Prob. 10.6PCh. 10 - Prob. 10.7PCh. 10 - Prob. 10.8PCh. 10 - 10-9 Is there any difference between vanillin made...Ch. 10 - Prob. 10.10P
Ch. 10 - 10-11 What important experiment did Wohler carry...Ch. 10 - Prob. 10.12PCh. 10 - Prob. 10.13PCh. 10 - Prob. 10.14PCh. 10 - 10-15 How many electrons are in the valence shell...Ch. 10 - 10-16 What is the relationship between the number...Ch. 10 - Prob. 10.17PCh. 10 - Prob. 10.18PCh. 10 - 10-19 Write Lewis structures for these ions. (a)...Ch. 10 - 10-20 Why are the following molecular formulas...Ch. 10 - 10-21 Explain how to use the valence-shell...Ch. 10 - 10-22 Suppose you forget to take into account the...Ch. 10 - Suppose you forget to take into account the...Ch. 10 - 10-24 Use the VSEPR model to predict the bond...Ch. 10 - Prob. 10.25PCh. 10 - Prob. 10.26PCh. 10 - 10-27 What is meant by the term functional group?Ch. 10 - 10-28 List three reasons why functional groups are...Ch. 10 - Prob. 10.29PCh. 10 - Prob. 10.30PCh. 10 - Prob. 10.31PCh. 10 - 10-32 Draw a structural formula for the one...Ch. 10 - 10-33 What is the meaning of the term tertiary (...Ch. 10 - Prob. 10.34PCh. 10 - Draw structural formulas for each of the...Ch. 10 - 10-36 Draw structural formulas for the six ketones...Ch. 10 - 10-37 Draw structural formulas for the eight...Ch. 10 - Prob. 10.38PCh. 10 - 10-39 (Chemical Connections 10A) How was Taxol...Ch. 10 - Prob. 10.40PCh. 10 - Prob. 10.41PCh. 10 - Silicon is immediately below carbon in Group 4A of...Ch. 10 - 10-43 Phosphorus is immediately below nitrogen in...Ch. 10 - Draw the structure for a compound with the...Ch. 10 - 10-45 Draw structural formulas for the eight...Ch. 10 - Prob. 10.46PCh. 10 - 10-47 Which of these covalent bonds are polar, and...Ch. 10 - Of the bonds in Problem 10-47, which is the most...Ch. 10 - Prob. 10.49PCh. 10 - Prob. 10.50PCh. 10 - Following is a structural formula for naphthalene....Ch. 10 - Prob. 10.52PCh. 10 - Prob. 10.53PCh. 10 - Urea, (NH.,)2CO, is used in plastics and in fertil...Ch. 10 - Prob. 10.55PCh. 10 - Prob. 10.56PCh. 10 - Aspirin is prepared by the reaction of salicylic-...Ch. 10 - Following is the structural formula of acetamide....Ch. 10 - Prob. 10.59P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- curved arrows are used to illustrate the flow of electrons. using the provided starting and product structures, draw the cured electron-pushing arrows for thw following reaction or mechanistic steps. be sure to account for all bond-breaking and bond making stepsarrow_forwardUsing the graphs could you help me explain the answers. I assumed that both graphs are proportional to the inverse of time, I think. Could you please help me.arrow_forwardSynthesis of Dibenzalacetone [References] Draw structures for the carbonyl electrophile and enolate nucleophile that react to give the enone below. Question 1 1 pt Question 2 1 pt Question 3 1 pt H Question 4 1 pt Question 5 1 pt Question 6 1 pt Question 7 1pt Question 8 1 pt Progress: 7/8 items Que Feb 24 at You do not have to consider stereochemistry. . Draw the enolate ion in its carbanion form. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. ⚫ Separate multiple reactants using the + sign from the drop-down menu. ? 4arrow_forward
- Shown below is the mechanism presented for the formation of biasplatin in reference 1 from the Background and Experiment document. The amounts used of each reactant are shown. Either draw or describe a better alternative to this mechanism. (Note that the first step represents two steps combined and the proton loss is not even shown; fixing these is not the desired improvement.) (Hints: The first step is correct, the second step is not; and the amount of the anhydride is in large excess to serve a purpose.)arrow_forwardHi I need help on the question provided in the image.arrow_forwardDraw a reasonable mechanism for the following reaction:arrow_forward
- Draw the mechanism for the following reaction: CH3 CH3 Et-OH Et Edit the reaction by drawing all steps in the appropriate boxes and connecting them with reaction arrows. Add charges where needed. Electron-flow arrows should start on the electron(s) of an atom or a bond and should end on an atom, bond, or location where a new bond should be created. H± EXP. L CONT. י Α [1] осн CH3 а CH3 :Ö Et H 0 N о S 0 Br Et-ÖH | P LL Farrow_forward20.00 mL of 0.150 M NaOH is titrated with 37.75 mL of HCl. What is the molarity of the HCl?arrow_forward20.00 mL of 0.025 M HCl is titrated with 0.035 M KOH. What volume of KOH is needed?arrow_forward
- 20.00 mL of 0.150 M NaOH is titrated with 37.75 mL of HCl. What is the molarity of the HCl?arrow_forward20.00 mL of 0.025 M HCl is titrated with 0.035 M KOH. What volume of KOH is needed?arrow_forward20.00 mL of 0.150 M HCl is titrated with 37.75 mL of NaOH. What is the molarity of the NaOH?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning


General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Acid-Base Titration | Acids, Bases & Alkalis | Chemistry | FuseSchool; Author: FuseSchool - Global Education;https://www.youtube.com/watch?v=yFqx6_Y6c2M;License: Standard YouTube License, CC-BY