
Concept explainers
State whether the following functions are acceptable wavefunctions over the range given. If they are not, explain why not.
(a)
(b)
(c)
(d) The function that looks like this:
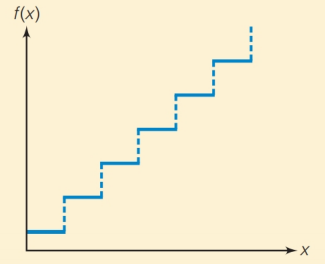
(e) The function that looks like this:
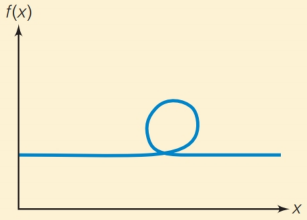
Trending nowThis is a popular solution!

Chapter 10 Solutions
PHYSICAL CHEMISTRY-STUDENT SOLN.MAN.
- Hi can you please help me solve this problem? thank youarrow_forwardAn electrode process takes place at a metal-solution interface. Indicate the current condition that must be met for Faradaic rectification to occur.arrow_forwardAt a metal-solution interface, an electron is exchanged, and the symmetry factor beta < 0.5 is found in the Butler-Volmer equation. What does this indicate?arrow_forward
 Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

