4) ½N2(g) + 02(g)= NO2(g) Kc = 1.04 x 10 N2(g) + 202(g) =N204(g) Kc=7.42 x 1018 %3D Calculate Kc for each of the following. (a) 2NO2(g)= N2(g) + 202(g) (b) N2(g) + 202(g) N2O4(g) (c) 2NO2(g) = N2O4(g)
4) ½N2(g) + 02(g)= NO2(g) Kc = 1.04 x 10 N2(g) + 202(g) =N204(g) Kc=7.42 x 1018 %3D Calculate Kc for each of the following. (a) 2NO2(g)= N2(g) + 202(g) (b) N2(g) + 202(g) N2O4(g) (c) 2NO2(g) = N2O4(g)
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
100%
Please explain step by step in neat hand writing so I can understand

Transcribed Image Text:**Chemical Equilibrium Question**
In this exercise, you will calculate the equilibrium constant, \( K_c \), for the following chemical reactions based on provided data.
**Given Reactions:**
1. \( \frac{1}{2}\text{N}_2(g) + \text{O}_2(g) \rightleftharpoons \text{NO}_2(g) \)
- \( K_c = 1.04 \times 10^{-3} \)
2. \( \text{N}_2(g) + 2\text{O}_2(g) \rightleftharpoons \text{N}_2\text{O}_4(g) \)
- \( K_c = 7.42 \times 10^{-18} \)
**Tasks:**
Calculate \( K_c \) for each of the following reactions:
(a) \( 2\text{NO}_2(g) \rightleftharpoons \text{N}_2(g) + 2\text{O}_2(g) \)
(b) \( \text{N}_2(g) + 2\text{O}_2(g) \rightleftharpoons \text{N}_2\text{O}_4(g) \)
(c) \( 2\text{NO}_2(g) \rightleftharpoons \text{N}_2\text{O}_4(g) \)
**Note:** Assume that the reactions are at equilibrium and that the provided equilibrium constants are applicable. Use principles of chemical equilibrium to determine the relationship between the given and calculated \( K_c \) values.
Expert Solution
Step 1
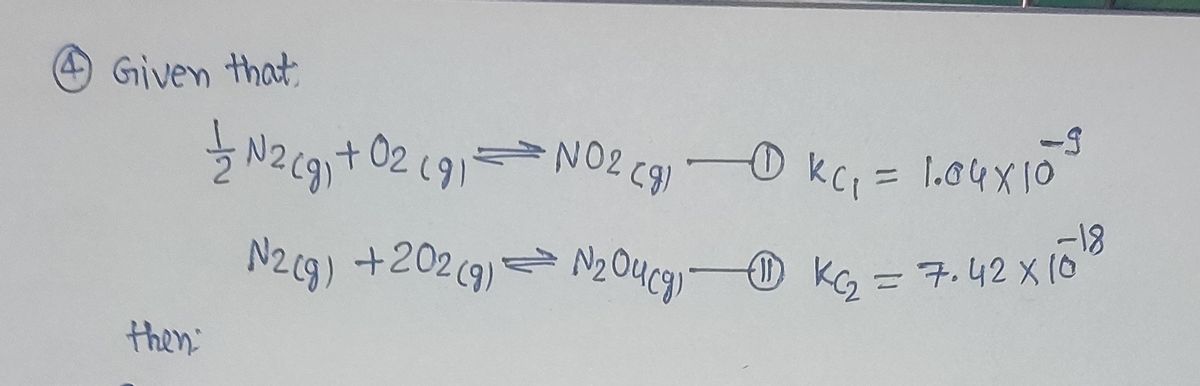
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY