
Organic Chemistry
11th Edition
ISBN: 9781118133576
Author: T. W. Graham Solomons, Craig Fryhle
Publisher: Wiley, John & Sons, Incorporated
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter SRP, Problem 20P
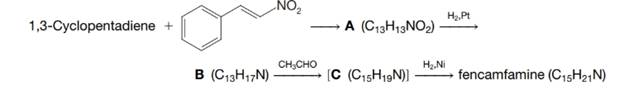
Outlined here is the synthesis of a central nervous system stimulant called fencamfamine. Provide structural formulas for each intermediate and for fencamfamine itself:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Don't used hand raiting
Don't used hand raiting
Don't used hand raiting
Chapter SRP Solutions
Organic Chemistry
Ch. SRP - 1. Arrange the compounds of each of the following...Ch. SRP - 2. Arrange the compounds of each of the following...Ch. SRP - Predict the final product from each of the...Ch. SRP - Prob. 4PCh. SRP - Write detailed mechanisms for each of the...Ch. SRP - Prob. 6PCh. SRP - Prob. 7PCh. SRP - Give stereochemical structures for compounds AD:Ch. SRP - Prob. 9PCh. SRP - The remaining steps in the industrial synthesis of...
Ch. SRP - Prob. 11PCh. SRP - Prob. 12PCh. SRP - Prob. 13PCh. SRP - Prob. 14PCh. SRP - 17. Show how you would modify the synthesis given...Ch. SRP - Prob. 16PCh. SRP - Give structures for compounds AD. Compound D gives...Ch. SRP - The tranquilizing drug meprobamate (Equanil or...Ch. SRP - Prob. 19PCh. SRP - 22. Outlined here is the synthesis of a central...Ch. SRP - 23. What are compounds A and B? Compound B has a...Ch. SRP - Prob. 22PCh. SRP - Prob. 26PCh. SRP - 28. Compound Y shows prominent IR absorption...Ch. SRP - Prob. 24PCh. SRP - Consider this reaction involving peracetic acid:...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Why are the top predators in food chains most severely affected by pesticides such as DDT?
Campbell Essential Biology with Physiology (5th Edition)
According to the logistic growth equation dNdt=rN(KN)K (A) the number of individuals added per unit time is gre...
Campbell Biology (11th Edition)
A 2.144-g sample of phosgene, a compound used as a chemical warfare agent during World War I, contains 0.260 g...
Chemistry: The Central Science (14th Edition)
For each reaction, calculate how many moles of product from when 1.75 mol of the reactant in color completely r...
Introductory Chemistry (6th Edition)
All of the following terms can appropriately describe humans except: a. primary consumer b. autotroph c. hetero...
Human Biology: Concepts and Current Issues (8th Edition)
3. What is free-fall, and why does it make you weightless? Briefly describe why astronauts are weightless in th...
The Cosmic Perspective (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- If a high molecular weight linear polyethylene is chlorinated by inducing the substitution of chlorine atoms by hydrogen, if 5% of all hydrogen atoms are replaced, what approximate percentage of chlorine by weight would the product have?arrow_forwardO Macmillan Learning Chemistry: Fundamentals and Principles Davidson presented by Macmillan Learning Poly(ethylene terephthalate), known as PET or industrially as Dacron, is a polyester synthesized through a condensation reaction between two bifunctional monomers. The monomers, ethylene glycol and terepthalic acid, are given. Add bonds and remove atoms as necessary to show the structure of a two repeat unit portion of a longer polymer chain of PET. You may need to zoom out to see the complete structure of all four monomer units. Select Draw / || | C H 0 3 © Templates More ° ° ° || C CC - OH HO OH HOC - C Erase CC OH HO C C 〃 C H₂ Q2Qarrow_forwardc) + H₂Oarrow_forward
- 으 b) + BF. 3 H2Oarrow_forwardQ4: Draw the product of each Lewis acid-bas reaction. Label the electrophile and nucleophile. b) S + AICI 3 + BF 3arrow_forwardQ1 - What type(s) of bonding would be expected for each of the following materials: solid xenon, calcium fluoride (CaF2), bronze, cadmium telluride (CdTe), rubber, and tungsten? Material solid xenon CaF2 bronze CdTe rubber tungsten Type(s) of bonding Q2- If the atomic radius of lead is 0.175 nm, calculate the volume of its unit cell in cubic meters.arrow_forward
- Determine the atomic packing factor of quartz, knowing that the number of Si atoms per cm3 is 2.66·1022 and that the atomic radii of silicon and oxygen are 0.038 and 0.117 nm.arrow_forwardUse the following data for an unknown gas at 300 K to determine the molecular mass of the gas.arrow_forward2. Provide a complete retrosynthetic analysis and a complete forward synthetic scheme to make the following target molecule from the given starting material. You may use any other reagents necessary. Brarrow_forward
- 146. Use the following data for NH3(g) at 273 K to determine B2p (T) at 273 K. P (bar) 0.10 0.20 0.30 0.40 0.50 0.60 (Z -1)/10-4 1.519 3.038 4.557 6.071 7.583 9.002 0.70 10.551arrow_forward110. Compare the pressures given by (a) the ideal gas law, (b) the van der Waals equation, and (c) the Redlic-Kwong equation for propane at 400 K and p = 10.62 mol dm³. The van der Waals parameters for propane are a = 9.3919 dm6 bar mol-2 and b = 0.090494 dm³ mol−1. The Redlich-Kwong parameters are A = 183.02 dm bar mol-2 and B = 0.062723 dm³ mol-1. The experimental value is 400 bar.arrow_forwardResearch in surface science is carried out using stainless steel ultra-high vacuum chambers with pressures as low as 10-12 torr. How many molecules are there in a 1.00 cm3 volume at this pressure and at a temperature of 300 K? For comparison, calculate the number of molecules in a 1.00 cm3 volume at atmospheric pressure and room temperature. In outer space the pressure is approximately 1.3 x 10-11 Pa and the temperature is approximately 2.7 K (determined using the blackbody radiation of the universe). How many molecules would you expect find in 1.00 cm3 of outer space?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY