
Organic Chemistry
11th Edition
ISBN: 9781118133576
Author: T. W. Graham Solomons, Craig Fryhle
Publisher: Wiley, John & Sons, Incorporated
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter SRP, Problem 8P
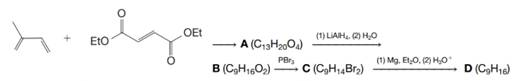
Give stereochemical structures for compounds A–D:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Use the data below from an electron impact mass spectrum of a pure compound to deduce its structure. Draw your structure in the
drawing window.
Data selected from the NIST
WebBook,
https://webbook.nist.gov/chemistry/
m/z
Relative intensity
31
0.5
30
26
29
22
28
100
27
33
26
23
15
4
• You do not have to consider stereochemistry.
You do not have to explicitly draw H atoms.
• In cases where there is more than one answer, just draw one.
妊
n
?
Previous
Next
for this question.
Write the molecular formula for a compound with the possible elements C, H, N and O that exhibits a molecular ion at M+ = 98.1106.
Exact Masses of the Most Abundant Isotope of
Selected Elements
Isotope Natural abundance (%) Exact mass
1H
99.985
1.008
12C
98.90
12.000
14N
99.63
14.003
160
99.76
15.995
Molecular formula
(In the order CHNO, with no subscripts)
PLEASE READ!!! I DONT WANT EXAMPLES, I DONT WANT WORDS OR PARAGRAPHS!!! PLEASE I UNDERSTAND THE BASICS BUT THIS IS AN EXCEPTION THAT EVEN THE INTERNET CANT HELP!!!!
THIS IS THE THIRD TIME I'VE SENT THOSE QUESTIONS SO PLEASE DONT RESEND THE SAME STUFF, ITS NOT HELPING ME!!!
I ALSO ALREADY TRIED TO DRAW THE MECHANISM MYSELF, SO IF ITS RIGHT PLEASE TELL ME OR TELL ME WHAT I HAVE TO CHANGE!!!
First image: I have to SHOW (DRAWING) the mechanism (with arows and structures of molecules) NOT WORDS PLEASE! of the reaction at the bottom. Also I have to show by mecanism why the reaction wouldn't work if the alcohol was primary
Second image: I have to show the mechanism (IMAGE) (with arrows and structures of the molecules) NOT WORDS PLEASE !! for the reaction on the left, where the alcohol A is added fast in one portion
HOMEWORK, NOT EXAM!!
ALL DETAILS ARE IN THE IMAGES PLEASE LOOK AT THE IMAGES, DONT LOOK AT THE AI GENERATED TEXT!!!
Chapter SRP Solutions
Organic Chemistry
Ch. SRP - 1. Arrange the compounds of each of the following...Ch. SRP - 2. Arrange the compounds of each of the following...Ch. SRP - Predict the final product from each of the...Ch. SRP - Prob. 4PCh. SRP - Write detailed mechanisms for each of the...Ch. SRP - Prob. 6PCh. SRP - Prob. 7PCh. SRP - Give stereochemical structures for compounds AD:Ch. SRP - Prob. 9PCh. SRP - The remaining steps in the industrial synthesis of...
Ch. SRP - Prob. 11PCh. SRP - Prob. 12PCh. SRP - Prob. 13PCh. SRP - Prob. 14PCh. SRP - 17. Show how you would modify the synthesis given...Ch. SRP - Prob. 16PCh. SRP - Give structures for compounds AD. Compound D gives...Ch. SRP - The tranquilizing drug meprobamate (Equanil or...Ch. SRP - Prob. 19PCh. SRP - 22. Outlined here is the synthesis of a central...Ch. SRP - 23. What are compounds A and B? Compound B has a...Ch. SRP - Prob. 22PCh. SRP - Prob. 26PCh. SRP - 28. Compound Y shows prominent IR absorption...Ch. SRP - Prob. 24PCh. SRP - Consider this reaction involving peracetic acid:...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Which of the roll owing compounds have a dipole moment of zero?
Organic Chemistry (8th Edition)
Flask A contains yeast cells in glucose-minimal salts broth incubated at 30C with aeration. Flask B contains ye...
Microbiology: An Introduction
6.1 State the number of electrons that be must be lost by atoms of each of the following to achieve a stable el...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
l. FIGURE Q26.1 shows the x-component of as a function of x. Draw a graph of V versus x in this same region of ...
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
8. A human maintaining a vegan diet (containing no animal products) would be a:
a. producer
b. primary consume...
Human Biology: Concepts and Current Issues (8th Edition)
Identify each of the following reproductive barriers as prezygotic or postzygotic. a. One lilac species lives o...
Campbell Essential Biology with Physiology (5th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Write the molecular formula for a compound with the possible elements C, H, N and O that exhibits a molecular ion at M+ = 85.0899. Exact Masses of the Most Abundant Isotope of Selected Elements Isotope Natural abundance (%) Exact mass 1H 99.985 1.008 12C 98.90 12.000 14N 99.63 14.003 160 99.76 15.995 Molecular formula (In the order CHNO, with no subscripts)arrow_forwardUse the data below from an electron impact mass spectrum of a pure compound to deduce its structure. Draw your structure in the drawing window. Data selected from the NIST WebBook, https://webbook.nist.gov/chemistry/ m/z Relative intensity 59 3.0 58 64 43 100 15 23 • You do not have to consider stereochemistry. •You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one. + n[] 85 // ? CH4 Previous Nextarrow_forwardWrite the molecular formula for a compound with the possible elements C, H, N and O that exhibits a molecular ion at M* = 128.0632. Exact Masses of the Most Abundant Isotope of Selected Elements Isotope Natural abundance (%) Exact mass 1H 99.985 12C 98.90 14N 99.63 160 99.76 Molecular formula 1.008 12.000 14.003 15.995 (In the order CHNO, with no subscripts)arrow_forward
- Can I please get help with this? And can I please the lowest possible significant number?arrow_forwardWhat is the molar mass of a gas that takes three times longer to effuse than helium?arrow_forwardFirst image: I have to show the mecanism (with arows and structures) of the reaction at the bottom. Also I have to show by mecanism why the reaction wouldn't work if the alcohol was primary Second image: I have to show the mecanism (with arrows and structures) for the reaction on the left, where the alcohol A is added fast in one portion its not an examarrow_forward
- what is the skeletal structure of a tertiary alkyl fluoride with six carbon atoms and no rings.arrow_forwardOne step of glycolysis is a retro-aldol reaction (aldolase) to produce ATP.Below is the aldol reaction of the equilibrium. Show the mechanism for the base catalyzed reaction. *see imagearrow_forwardProvide the missing information. *see imagearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

How to Design a Total Synthesis; Author: Chemistry Unleashed;https://www.youtube.com/watch?v=9jRfAJJO7mM;License: Standard YouTube License, CC-BY