
a)
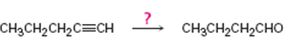
Interpretation:
How to synthesize butanal from 1-pentyne is to be shown.
Concept introduction:
To show:
How to synthesize butanal from 1-pentyne.
b)
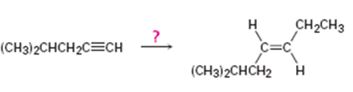
Interpretation:
How to synthesize trans- 6-methyl-3 heptene from 4-methyl-1-pentyne is to be shown.
Concept introduction:
Higher alkynes can be prepared first by converting the lower alkyne in to an alkynide by reacting with NaNH2 in liquid NH3 followed by the reaction of the alkynide with
To show:
How to synthesize trans- 6-methyl-3 heptene from 4-methyl-1-pentyne.
Want to see the full answer?
Check out a sample textbook solution
Chapter 9 Solutions
EBK ORGANIC CHEMISTRY
- In reactions whose kinetic equation is v = k[A]m, the rate coefficient k is always positive. Is this correct?arrow_forwardIf the concentration of A decreases exponentially with time, what is the rate equation? (A). -d[A] (B). dt d[A] = k[A] e-kt dtarrow_forwardGiven the first-order reaction: aA → products. State its kinetic equation.arrow_forward
- The following chemical structure represents a molecule of what molecular formula?arrow_forwardWhich region(s) of the following phospholipid is/are hydrophobic? RO I hydro-water phobic-dislikes = Hydrophobic dislikes water ○ I only Il only I and III only II and IV only O II, III, and IV only III || IVarrow_forwardPredict the product of the following reactions: O 0= excess Х Кон ОН H+ H+ Iarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

