
Concept explainers
a)
Interpretation:
The configurational stereochemistry of the molecules to be determined.
Answer to Problem 26VC
The Fischer projection follows as
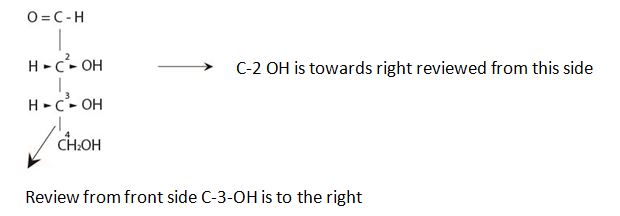
It is D sugar the molecule is a retrose. Elclose or eldotetrose, is 4-carbon elclose.
Explanation of Solution
Concept strategy: The Fischer projection of the given monosaccharide is drawn vertically, by rotating the molecule anticlockwise 90° so that the carbonyl
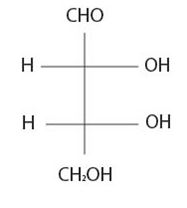
Review from front side C-3-OH is to the right By convention the molecule has the C-3 hyduxyl at the right. So it is D sugar the molecule is a retrose. Elclose or eldotetrose, is 4-carbon elclose.
Based on the Fischer projection formula for the given sugars it is a B-D-glucopyranose monosaccharide.
b)
Interpretation:
The configurational stereochemistry of the molecules to be determined.
Answer to Problem 26VC
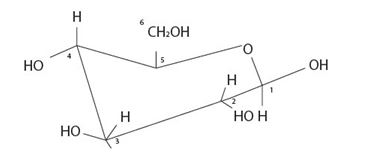
The given model is the cyclic structures of an aldohexose in six membered pyranose form.
Strategy: We redraw the model as
Explanation of Solution
By convention, the terminal -CH2OH group is on the top of the chair Pyranose structure. Thus it is a D sugar. The molecule is an aldohexose is B-D-glucopyranose, all the –OH groups are equatorial (and more stable due to minimum repulsion) conformation.
Based on the Fischer projection formula for the given sugars it is a B-D-glucopyranose monosaccharide.
Want to see more full solutions like this?
Chapter 25 Solutions
EBK ORGANIC CHEMISTRY
- On what basis are Na and Nb ranked against each other?arrow_forwardStep 1: add a curved arrow. Select Draw Templates More / " C H Br 0 Br : :o: Erase H H H H Q2Q Step 2: Draw the intermediates and a curved arrow. Select Draw Templates More MacBook Air / " C H Br 0 9 Q Erase 2Qarrow_forwardO Macmillan Learning Question 23 of 26 > Stacked Step 7: Check your work. Does your synthesis strategy give a substitution reaction with the expected regiochemistry and stereochemistry? Draw the expected product of the forward reaction. - - CN DMF MacBook Air Clearly show stereochemistry. Questionarrow_forward
- NH2 1. CH3–MgCl 2. H3O+ ? As the lead product manager at OrganometALEKS Industries, you are trying to decide if the following reaction will make a molecule with a new C - C bond as its major product: If this reaction will work, draw the major organic product or products you would expect in the drawing area below. If there's more than one major product, you can draw them in any arrangement you like. Be sure you use wedge and dash bonds if necessary, for example to distinguish between major products with different stereochemistry. If the major products of this reaction won't have a new C - C bond, just check the box under the drawing area and leave it blank. Click and drag to start drawing a structure. This reaction will not make a product with a new C - C bond. Х ☐: Carrow_forwardPredict the major products of this organic reaction. If there will be no major products, check the box under the drawing area instead. No reaction. : + Х è OH K Cr O 2 27 2 4' 2 Click and drag to start drawing a structure.arrow_forwardLaminar compounds are characterized by havinga) a high value of the internal surface of the solid.b) a high adsorption potential.arrow_forward
