
a)
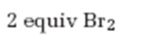
Interpretation:
The product formed when 2-hexyne reacts with 2 equivalents of bromine.
Concept introduction:
In addition reactions,
To give:
The product formed when 2-hexyne reacts with two molar equivalents of bromine.
b)
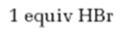
Interpretation:
The product formed when 2-hexyne reacts with 1 equivalent of HBr is to be predicted.
Concept introduction:
In addition reactions, alkynes when treated with two molar equivalents of a reagent yield the derivatives of the corresponding alkane as the product. With one molar equivalent of a reagent they yield a derivative of the corresponding alkene as the product. The addition to unsymmetrical alkenes follows Markovnikov regiochemistry. The negative part of the reagent adds on to the more highly substituted carbon in triple bond.
To predict:
The product formed when 2-hexyne reacts with 1 equivalent of HBr.
c)

Interpretation:
The product formed when 2-hexyne reacts with 1 equivalent of HBr is to be predicted.
Concept introduction:
In addition reactions, alkynes when treated with excess amount of a reagent yield the derivatives of the corresponding alkane as the product. The addition to unsymmetrical alkenes follows Markovnikov regiochemistry. The negative part of the reagent adds on to the more highly substituted carbon in triple bond.
To predict:
The product formed when 2-hexyne reacts with excess of HBr.
d)
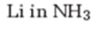
Interpretation:
The product formed when 2-hexyne reacts with Li in ammonia.
Concept introduction:
Alkynes can be reduced by treatment with hydrogen in the presence of a catalyst. The hydrogenation occurs with anti stereochemistry to give a trans- alkene as product if Li in ammonia is used.
To predict:
The product formed when 2-hexyne reacts with Li in ammonia.
e)
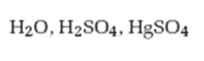
Interpretation:
The product formed when 2-hexyne reacts with H2O, H2SO4 in the presence of HgSO4 is to be given.
Concept introduction:
When treated with H2O, H2SO4 in the presence of HgSO4 alkynes get hydrated to produce enols. The addition of water to unsymmetrical alkynes follows Markonikov regiochemistry while it cannot be applied in the case of symmetrical alkynes. The enols produced then undergo tautomerization to give a
To predict:
The product formed when 2-hexyne reacts with H2O, H2SO4 in the presence of HgSO4.
Trending nowThis is a popular solution!

Chapter 9 Solutions
OWLv2 with Student Solutions Manual eBook, 4 terms (24 months) Printed Access Card for McMurry's Organic Chemistry, 9th
- Propose a synthesis pathway for the following transformations. b) c) d)arrow_forwardThe rate coefficient of the gas-phase reaction 2 NO2 + O3 → N2O5 + O2 is 2.0x104 mol–1 dm3 s–1 at 300 K. Indicate whether the order of the reaction is 0, 1, or 2.arrow_forward8. Draw all the resonance forms for each of the following molecules or ions, and indicate the major contributor in each case, or if they are equivalent. (4.5 pts) (a) PH2 سمةarrow_forward
- 3. Assign absolute configuration (Rors) to each chirality center. a. H Nitz C. он b. 0 H-C. C H 7 C. ་-4 917-417 refs H 1つ ८ ડુ d. Но f. -2- 01 Ho -OH 2HNarrow_forwardHow many signals do you expect in the H NMR spectrum for this molecule? Br Br Write the answer below. Also, in each of the drawing areas below is a copy of the molecule, with Hs shown. In each copy, one of the H atoms is colored red. Highlight in red all other H atoms that would contribute to the same signal as the H already highlighted red. Note for advanced students: In this question, any multiplet is counted as one signal. Number of signals in the 'H NMR spectrum. For the molecule in the top drawing area, highlight in red any other H atoms that will contribute to the same signal as the H atom already highlighted red. If no other H atoms will contribute, check the box at right. No additional Hs to color in top molecule For the molecule in the bottom drawing area, highlight in red any other H atoms that will contribute to the same signal as the H atom already highlighted red. If no other H atoms will contribute, check the box at right. No additional Hs to color in bottom moleculearrow_forwardIn the drawing area below, draw the major products of this organic reaction: 1. NaOH ? 2. CH3Br If there are no major products, because nothing much will happen to the reactant under these reaction conditions, check the box under the drawing area instead. No reaction. Click and drag to start drawing a structure. ☐ : A คarrow_forward
- Predict the major products of the following organic reaction: NC Δ ? Some important Notes: • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to draw bonds carefully to show important geometric relationships between substituents. Note: if your answer contains a complicated ring structure, you must use one of the molecular fragment stamps (available in the menu at right) to enter the ring structure. You can add any substituents using the pencil tool in the usual way. Click and drag to start drawing a structure. Х аarrow_forwardPredict the major products of this organic reaction. Be sure you use dash and wedge bonds to show stereochemistry where it's important. + ☑ OH 1. TsCl, py .... 文 P 2. t-BuO K Click and drag to start drawing a structure.arrow_forwardConsider this organic reaction: ( Draw the major products of the reaction in the drawing area below. If there won't be any major products, because this reaction won't happen at a significant rate, check the box under the drawing area instead. Click and drag to start drawing a structure. Х : а ค 1arrow_forward
- In the drawing area below, draw the major products of this organic reaction: If there are no major products, because nothing much will happen to the reactant under these reaction conditions, check the box under the drawing area instead. 1. NaH 2. CH3Br ? Click and drag to start drawing a structure. No reaction. : ☐ Narrow_forward+ Predict the major product of the following reaction. : ☐ + ☑ ค OH H₂SO4 Click and drag to start drawing a structure.arrow_forwardConsider this organic reaction: ... OH CI Draw the major products of the reaction in the drawing area below. If there won't be any major products, because this reaction won't happen at a significant rate, check the box under the drawing area instead. ☐ No Reaction. Click and drag to start drawing a structure. : аarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

