
OWLv2 with Student Solutions Manual eBook, 4 terms (24 months) Printed Access Card for McMurry's Organic Chemistry, 9th
9th Edition
ISBN: 9781305671874
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 9.SE, Problem 25MP
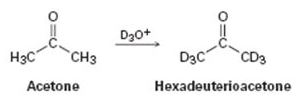
Reaction of acetone with D3O+ yields hexadeuterioacetone. That is, all the hydrogens in acetone are exchanged for deuterium. Review the mechanism of mercuric-ion-catalyzed
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Using Benzene as starting materid show
how each of the Following molecules Contel
Ve syntheswed
CHI
9.
b
-50311
с
CHY
503H
Ночто
d.
อ
•NOV
e
11-0-650
NO2
The molecule PYRIDINE,
6th electrons and is therefore aromatre
and is Assigned the Following structure
contering
Since aromatk moleculoy undergo electrophilic
anomatic substitution, Pyridine shodd undergo
The Following reaction
+ HNO3
12504
a. write all of the possible Mononitration Products
that could Result From this reaction
18. Bared upon the reaction mechanison determime
which of these producty would be the major
Product of the hegetion
a. Explain Why electron withdrawing groups
tend to be meta-Directors. Your answer Should
lyclude all apropriate. Resonance contributing
Structures
fo. Explain why -ll is an outho -tura
drccton even though chlorine has a very High
Electronegativity
Chapter 9 Solutions
OWLv2 with Student Solutions Manual eBook, 4 terms (24 months) Printed Access Card for McMurry's Organic Chemistry, 9th
Ch. 9.1 - Prob. 1PCh. 9.1 - Prob. 2PCh. 9.3 - What products would you expect from the following...Ch. 9.4 - Prob. 4PCh. 9.4 - Prob. 5PCh. 9.4 - Prob. 6PCh. 9.4 - Prob. 7PCh. 9.5 - Using any alkyne needed, how would you prepare the...Ch. 9.7 - The pKa of acetone, CH3COCH3, is 19.3. Which of...Ch. 9.8 - Prob. 10P
Ch. 9.8 - Prob. 11PCh. 9.9 - Show the terminal alkyne and alkyl halide from...Ch. 9.9 - Beginning with acetylene and any alkyl halide...Ch. 9.SE - Name the following alkynes, and predict the...Ch. 9.SE - From what alkyne might each of the following...Ch. 9.SE - Prob. 16VCCh. 9.SE - The following cycloalkyne is too unstable to...Ch. 9.SE - Prob. 18MPCh. 9.SE - Assuming that strong acids add to alkynes in the...Ch. 9.SE - Prob. 20MPCh. 9.SE - Prob. 21MPCh. 9.SE - Prob. 22MPCh. 9.SE - Prob. 23MPCh. 9.SE - Prob. 24MPCh. 9.SE - Reaction of acetone with D3O+ yields...Ch. 9.SE - Give IUPAC names for the following compounds:Ch. 9.SE - Draw structures corresponding to the following...Ch. 9.SE - Prob. 28APCh. 9.SE - Prob. 29APCh. 9.SE - Prob. 30APCh. 9.SE - Predict the products from reaction of l-hexyne...Ch. 9.SE - Prob. 32APCh. 9.SE - Prob. 33APCh. 9.SE - Propose structures for hydrocarbons that give the...Ch. 9.SE - Identify the reagents a-c in the following scheme:Ch. 9.SE - Prob. 36APCh. 9.SE - Prob. 37APCh. 9.SE - Prob. 38APCh. 9.SE - How would you carry out the following...Ch. 9.SE - Prob. 40APCh. 9.SE - Synthesize the following compounds using 1-butyne...Ch. 9.SE - Prob. 42APCh. 9.SE - Prob. 43APCh. 9.SE - Prob. 44APCh. 9.SE - Prob. 45APCh. 9.SE - A hydrocarbon of unknown structure has the formula...Ch. 9.SE - Compound A(C9H12) absorbed 3 equivalents of H2 on...Ch. 9.SE - Hydrocarbon A has the formula C12H8. It absorbs 8...Ch. 9.SE - Occasionally, a chemist might need to invert the...Ch. 9.SE - Prob. 50APCh. 9.SE - Prob. 51APCh. 9.SE - Prob. 52APCh. 9.SE - Prob. 53APCh. 9.SE - Prob. 54APCh. 9.SE - Prob. 55APCh. 9.SE - Prob. 56APCh. 9.SE - Prob. 57AP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 9. Write Me product as well as the reaction Mechanism For each of the Following Vanctions +H₂504 4.50+ T C. +212 Fellz 237 b. Praw the potential energy Diagrams For each OF Mese Rauctions and account For any differences that appear in the two potential Puergy Diagrams which of here two reactions 19 Found to be Reversable, Rationalice your answer based upon the venation mechanisms and the potential energy diagrams.arrow_forward9. Write Me product as well as the reaction Mechanism For each of the Following Veritious +H2504 4.50+ + 1/₂ Felly ◎+ 7 b. Praw he potential energy Diagrams For each OF Mese Ronctions and account for any differences that appeak in the two potential Puergy Diagramsarrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Incorrect, 3 attempts remaining 1. excess Br2, NaOH 2. neutralizing workup Qarrow_forward
- Given the electrode Pt | Ag | Ag+ (aq), describe it.arrow_forwardAt 25°C, the reaction Zn2+ + 2e ⇄ Zn has a normal equilibrium potential versus the saturated calomel electrode of -1.0048 V. Determine the normal equilibrium potential of Zn versus the hydrogen electrode.Data: The calomel electrode potential is E° = 0.2420 V versus the normal hydrogen electrode.arrow_forwardElectrochemistry. State the difference between E and E0.arrow_forward
- In an electrolytic cell, the positive pole is always assumed to be on the right side of the battery notation. Is that correct?arrow_forwardIn an electrolytic cell, the positive pole is always assumed to be on the right side of the battery. Is that correct?arrow_forwardCalculate the free energy of formation of 1 mol of Cu in cells where the electrolyte is 1 mol dm-3 Cu2+ in sulfate solution, pH 0. E° for the Cu2+/Cu pair in this medium is +142 mV versus ENH.Assume the anodic reaction is oxygen evolution.Data: EH2 = -0.059 pH (V) and EO2 = 1.230 - 0.059 pH (V); 2.3RT/F = 0.059 Varrow_forward
- If the normal potential for the Fe(III)/Fe(II) pair in acid at zero pH is 524 mV Hg/Hg2Cl2 . The potential of the saturated calomel reference electrode is +246 mV versus the NHE. Calculate E0 vs NHE.arrow_forwardGiven the galvanic cell whose scheme is: (-) Zn/Zn2+ ⋮⋮ Ag+/Ag (+). If we know the normal potentials E°(Zn2+/Zn) = -0.76V and E°(Ag+/Ag) = 0.799 V. Indicate the electrodes that are the anode and the cathode and calculate the E0battery.arrow_forwardIndicate the functions that salt bridges have in batteries.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License