
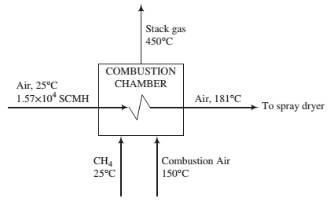
Methane is burned completely with 40% excess air. The methane enters the combustion chamber at 25°C, the combustion air enters at 150°C. and the stack gas [CO2, H2O(v), O2, N2] exits at 450°C. The chamber functions as a preheater for an air stream flowing in a pipe through the chamber to a spray dryer. The air enters the chamber at 25°C at a rate of 1.57 × 104m3(STP)/h and is heated to 181°C. All of the heat generated by combustion is used to heat the combustion products and the air going to the spray dryer (i.e., the combustion chamber may be considered adiabatic).
- Draw and completely label the process flow' diagram and perform a degree-of-freedom analysis.
Want to see the full answer?
Check out a sample textbook solution
Chapter 9 Solutions
Elementary Principles of Chemical Processes, Binder Ready Version
Additional Engineering Textbook Solutions
Starting Out With Visual Basic (8th Edition)
Concepts Of Programming Languages
Starting Out with Python (4th Edition)
SURVEY OF OPERATING SYSTEMS
Computer Science: An Overview (13th Edition) (What's New in Computer Science)
Starting Out with Java: From Control Structures through Data Structures (4th Edition) (What's New in Computer Science)
- The power out of an adiabatic steam turbine is 5 MW and the steam enters turbine at 2 MPa and velocity of 50 m/s, specific enthalpy (h) of 3248 kJ/kg. The elevation of the inlet is 10 m higher than at the datum. The vapor mixture exits at 15 kPa and a velocity of 180 m/s, specific enthalpy (h) of 2361.01 kJ/kg. The elevation of the exit is 6 m higher than at the datum. Let g = 9.81 m/s². Assuming the ideal gas model and R = 0.462 KJ/(kg.K). The steam specific heat ratio is 1.283. Calculate:arrow_forwardstep by step pleasearrow_forwardstep by step pleasearrow_forward
- step by steparrow_forwardThe power out of an adiabatic steam turbine is 5 MW and the steam enters turbine at 2 MPa and velocity of 50 m/s, specific enthalpy (h) of 3248 kJ/kg. The elevation of the inlet is 10 m higher than at the datum. The vapor mixture exits at 15 kPa and a velocity of 180 m/s, specific enthalpy (h) of 2361.01 kJ/kg. The elevation of the exit is 6 m higher than at the datum. Let g = 9.81 m/s². Assuming the ideal gas model and R = 0.462 KJ/(kg.K). The steam specific heat ratio is 1.283. Calculate:arrow_forwardThe power out of an adiabatic steam turbine is 5 MW and the steam enters turbine at 2 MPa and velocity of 50 m/s, specific enthalpy (h) of 3248 kJ/kg. The elevation of the inlet is 10 m higher than at the datum. The vapor mixture exits at 15 kPa and a velocity of 180 m/s, specific enthalpy (h) of 2361.01 kJ/kg. The elevation of the exit is 6 m higher than at the datum. Let g = 9.81 m/s². Assuming the ideal gas model and R = 0.462 KJ/(kg.K). The steam specific heat ratio is 1.283. Calculate:arrow_forward
- O Consider a 0.8 m high and 0.5 m wide window with thickness of 8 mm and thermal conductivity of k = 0.78 W/m °C. For dry day, the temperature of outdoor is -10 °C and the inner room temperature is 20°C. Take the heat transfer coefficient on the inner and outer surface of the window to be h₁ = 10 W/m² °C and h₂ = 40 W/m² °C which includes the effects of insulation. Determine:arrow_forwardCalculate the mass flow rate of the steam. Determine Cp and C₁ of steam.arrow_forwardstep by step pleasearrow_forward
- step by steparrow_forward4. Show that the fraction, F, of the energy released from a supercritical chain reaction that originates in the final m generations of the chain is given approximately by F= 1 km provided the total number of generations is large.arrow_forwardPLEASE SOLVE STEP BY STEP WITHOUT ARTIFICIAL INTELLIGENCE OR CHATGPT I don't understand why you use chatgpt, if I wanted to I would do it myself, I need to learn from you, not from being a d amn robot. SOLVE BY HAND STEP BY STEP A solution containing 7.5% sulfuric acid by weight at 70 °F is concentrated to 45% by weight by evaporating water. The concentrated solution and the water vapor exit the evaporator at 170 °F and 1 atm. Calculate the rate at which heat must be transferred to the evaporator to process 1500 lbm/hr of the feed solution to the evaporator. It is recommended to use the enthalpy-concentration diagram for sulfuric acid from Chapter 8 of Felder's book or an enthalpy-concentration diagram for sulfuric acid found in another unit operations book or chemical engineering manual such as Perry's.arrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





