
Concept explainers
Draw the product formed when
a.
b.
(a)
Interpretation: The product formed by the treatment of
Concept introduction: Alkyl chlorides are obtained by the reaction of
Answer to Problem 9.27P
The product formed by the treatment of
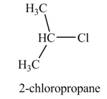
Explanation of Solution
The given reagent is
Alkyl chlorides are obtained by the reaction of
Thus, the product formed by the treatment of
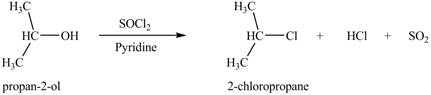
Figure 1
(a) The product formed by the treatment of
(b)
Interpretation: The product formed by the treatment of
Concept introduction: Alcohols are converted into alkyl tosylates by treatment with
Answer to Problem 9.27P
The product formed by the treatment of
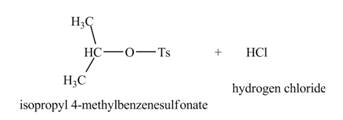
Explanation of Solution
The given reagent is
Alcohols are converted into alkyl tosylates by treatment with
Thus, the product formed by the treatment of
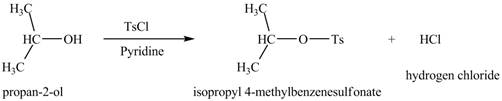
Figure 2
The product formed by the treatment of
(c)
Interpretation: The product formed by the treatment of
Concept introduction: Alcohols undergo dehydration reaction in the presence of strong acids like
Answer to Problem 9.27P
The product formed by the treatment of
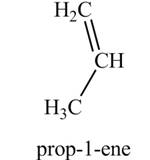
Explanation of Solution
The given reagent is
Alcohols undergo dehydration reaction in the presence of strong acids like
Thus, the product formed by the treatment of
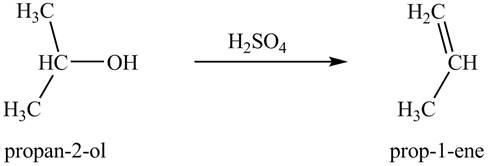
Figure 3
The product formed by the treatment of
(d)
Interpretation: The product formed by the treatment of
Concept introduction: The reaction of alcohols with halogen acids
Answer to Problem 9.27P
The product formed by the treatment of
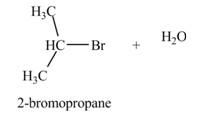
Explanation of Solution
The given reagent is
The reaction of alcohols with halogen acids
Thus, the product formed by the treatment of
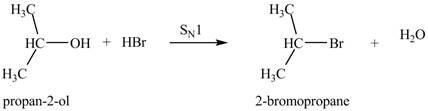
Figure 4
The product formed by the treatment of
(e)
Interpretation: The product formed by the treatment of
Concept introduction: Alkyl bromides are obtained by the reaction of
Answer to Problem 9.27P
The product formed by the treatment of
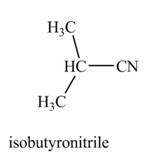
Explanation of Solution
The given reagents are
Alkyl bromides are obtained by the reaction of
Thus, the product formed by the treatment of
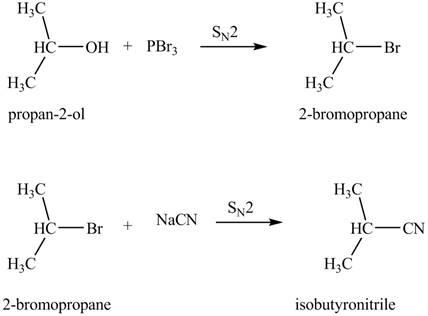
Figure 5
The product formed by the treatment of
(f)
Interpretation: The product formed by the treatment of
Concept introduction: Alcohols undergo dehydration reaction in the presence of
Answer to Problem 9.27P
The product formed by the treatment of
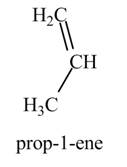
Explanation of Solution
The given reagent is
Alcohols undergo dehydration reaction in the presence of
Thus, the product formed by the treatment of
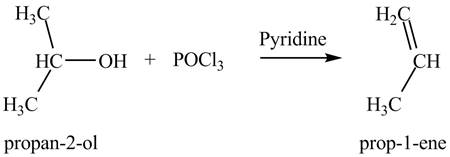
Figure 6
The product formed by the treatment of
Want to see more full solutions like this?
Chapter 9 Solutions
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
Additional Science Textbook Solutions
Biological Science (6th Edition)
Chemistry: Structure and Properties (2nd Edition)
Organic Chemistry (8th Edition)
Microbiology with Diseases by Body System (5th Edition)
Genetic Analysis: An Integrated Approach (3rd Edition)
The Cosmic Perspective (8th Edition)
- You're competing on a Great British television game show, and you need to bake a cake. The quantity for each ingredient is given in grams, but you haven't been given a kitchen scale. Which of these properties would correlate with the mass of a baking ingredient like eggs or milk? Check all that apply. depth of color viscosity volume densityarrow_forwardDraw a Lewis structure for each of the following species. Again, assign charges where appropriate. a. H-H¯ b. CH3-CH3 c. CH3+CH3 d. CH3 CH3 e. CH3NH3+CH3NH3 f. CH30-CH3O¯ g. CH2CH2 - h. HC2-(HCC) HC2 (HCC) i. H202×(HOOH) H₂O₂ (HOOH) Nortonarrow_forwardIs molecule 6 an enantiomer?arrow_forward
- Show work. Don't give Ai generated solutionarrow_forwardCheck the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. Molecule 1 Molecule 2 Molecule 3 ----||| Molecule 4 Molecule 5 Molecule 6 none of the above mm..arrow_forwardShow work. don't give Ai generated solutionarrow_forward
- Check the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. Molecule 1 Molecule 2 Molecule 3 ----||| Molecule 4 Molecule 5 Molecule 6 none of the above mm..arrow_forwardUse the vapor-liquid equilibrium data at 1.0 atm. for methanol-water (Table 2-8 ) for the following: If the methanol vapor mole fraction is 0.600, what is the methanol liquid mole fraction? Is there an azeotrope in the methanol-water system at a pressure of 1.0 atmospheres? If water liquid mole fraction is 0.350, what is the water vapor mole fraction? What are the K values of methanol and of water at a methanol mole fraction in the liquid of 0.200? What is the relative volatility αM-W at a methanol mole fraction in the liquid of 0.200?arrow_forwardCheck the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. || |II***** Molecule 1 | Molecule 4 none of the above Molecule 2 Molecule 3 Х mm... C ---||| *** Molecule 5 Molecule 6arrow_forward
- is SiBr4 Silicon (IV) tetra Bromine? is KClO2 potassium dihypochlorite ?arrow_forward"יוון HO" Br CI Check the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. Molecule 1 Molecule 2 Molecule 3 Br Br Br HO OH H CI OH ✓ Molecule 4 Molecule 5 Molecule 6 CI Br יייון H Br OH OH CI Br ☐ none of the above × Garrow_forwardUS2 Would this be Uranium (II) diSulfide?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





