
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
5th Edition
ISBN: 9781260170405
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 9, Problem 9.72P
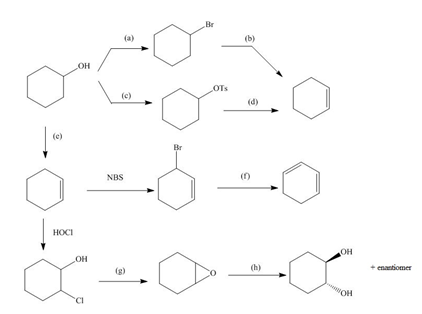
Identify the reagents (a–h) needed to carry out each reaction.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
ΗΝ,
Draw Final Product
C
cyclohexanone
pH 4-5
Edit Enamine
H3O+
CH3CH2Br
THF, reflux
H
Edit Iminium Ion
How many hydrogen atoms are connected to the indicated carbon atom?
Identify the compound with the longest carbon - nitrogen bond.
O CH3CH2CH=NH
O CH3CH2NH2
CH3CH2C=N
CH3CH=NCH 3
The length of all the carbon-nitrogen bonds are the same
Chapter 9 Solutions
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
Ch. 9 - Problem 9.1 Label each ether and alcohol in...Ch. 9 - Give the IUPAC name for each compound.Ch. 9 - Problem 9.3 Give the structure corresponding to...Ch. 9 - Name each of the following ethers.Ch. 9 - Name each epoxide.
a. (two ways) b. c. (two...Ch. 9 - Problem 9.6 Rank the following compounds in order...Ch. 9 - Which mechanism is favored by the use of crown...Ch. 9 - Problem 9.8 Draw the organic product of each...Ch. 9 - Prob. 9.9PCh. 9 - Problem 9.10 Draw the products of each reaction.
...
Ch. 9 - Problem 9.11 Draw the products formed when each...Ch. 9 - Prob. 9.12PCh. 9 - Problem 9.13 Draw the structure of each...Ch. 9 - What other alkene is also formed along with Y in...Ch. 9 - Prob. 9.15PCh. 9 - Explain why two substitution products are formed...Ch. 9 - Draw the products of each reaction. a. b. c.Ch. 9 - Problem 9.18 Draw the products of each reaction,...Ch. 9 - Problem 9.19 What is the major product formed...Ch. 9 - Prob. 9.20PCh. 9 - Prob. 9.21PCh. 9 - Problem 9.22 Draw the organic products formed in...Ch. 9 - Problem 9.23 Draw two steps to convert into each...Ch. 9 - Prob. 9.24PCh. 9 - Problem 9.25 Draw the products of each reaction,...Ch. 9 - Draw the products formed when (S)-butan-2-ol is...Ch. 9 - Draw the product formed when (CH3)2CHOH is treated...Ch. 9 - What alkyl halides are formed when each ether is...Ch. 9 - Explain why the treatment of anisole with HBr...Ch. 9 - Name each thiol.
a. b.
Ch. 9 - Draw the product of each reaction. ac b.d.Ch. 9 - Give the IUPAC name for each sulfide.
a. b.
Ch. 9 - Draw the product of each reaction.
a. b.
Ch. 9 - Prob. 9.34PCh. 9 - The cis and trans isomers of 2, 3-dimethyloxirane...Ch. 9 - Problem 9.36 Draw the product of each...Ch. 9 - 9.37 Name each compound depicted in the...Ch. 9 - Answer each question using the ball-and-stick...Ch. 9 - Prob. 9.39PCh. 9 - 9.40 Give IUPAC name for each...Ch. 9 - Prob. 9.41PCh. 9 - Prob. 9.42PCh. 9 - Prob. 9.43PCh. 9 - 9.44 Why is the boiling point of higher than...Ch. 9 - 9.45 Draw the organic product(s) formed when is...Ch. 9 - 9.46 What alkenes are formed when each alcohol is...Ch. 9 - Prob. 9.47PCh. 9 - 9.48 Draw the products of each reaction and...Ch. 9 - 9.49 Draw the product of the following reaction,...Ch. 9 - Prob. 9.50PCh. 9 - Prob. 9.51PCh. 9 - 9.52 Draw a stepwise mechanism for the following...Ch. 9 - 9.53 Although alcohol V gives a single alkene W...Ch. 9 - Prob. 9.54PCh. 9 - Prob. 9.55PCh. 9 - Prob. 9.56PCh. 9 - 9.57 Draw a stepwise, detailed mechanism for the...Ch. 9 - Prob. 9.58PCh. 9 - 9.59 Draw two different routes to each of the...Ch. 9 - Prob. 9.60PCh. 9 -
9.61 Draw the products formed when each ether is...Ch. 9 - 9.62 Draw a stepwise mechanism for each...Ch. 9 - Draw a stepwise, detailed mechanism for the...Ch. 9 - Prob. 9.64PCh. 9 - Draw the products of each reaction. a.c. b.d.Ch. 9 - When each halohydrin is treated with, a product of...Ch. 9 - Prob. 9.67PCh. 9 - Prob. 9.68PCh. 9 - Prob. 9.69PCh. 9 - Prob. 9.70PCh. 9 - Prepare each compound from cyclopentanol. More...Ch. 9 - 9.72 Identify the reagents (a–h) needed to carry...Ch. 9 - Prob. 9.73PCh. 9 - 9.74 Treatment of with affords compound A and ....Ch. 9 - Prob. 9.75PCh. 9 - Prob. 9.76PCh. 9 - 9.77 Draw a stepwise, detailed mechanism for the...Ch. 9 - 9.78 Dehydration of with affords
as a minor...Ch. 9 - Prob. 9.79PCh. 9 - 9.80 Draw a stepwise mechanism for the following...Ch. 9 -
9.81 Aziridines are heterocycles that contain an...
Additional Science Textbook Solutions
Find more solutions based on key concepts
6. How can you use the features found in each chapter?
Human Anatomy & Physiology (2nd Edition)
An obese 55-year-old woman consults her physician about minor chest pains during exercise. Explain the physicia...
Biology: Life on Earth with Physiology (11th Edition)
To test your knowledge, discuss the following topics with a study partner or in writing ideally from memory. Th...
HUMAN ANATOMY
Why are mutants used as test organisms in the Ames test?
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
Identify each of the following reproductive barriers as prezygotic or postzygotic. a. One lilac species lives o...
Campbell Essential Biology with Physiology (5th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Identify any polar covalent bonds in epichlorohydrin with S+ and 8- symbols in the appropriate locations. Choose the correct answer below. Η H's+ 6Η Η Η Η Η Ηδ Η Ο Ο HH +Η Η +Η Η Η -8+ CIarrow_forwardH H:O::::H H H HH H::O:D:D:H HH HH H:O:D:D:H .. HH H:O:D:D:H H H Select the correct Lewis dot structure for the following compound: CH3CH2OHarrow_forwardRank the following compounds in order of decreasing boiling point. ннннн -С-С-Н . н-с- ННННН H ΗΤΗ НННН TTTĪ н-с-с-с-с-о-н НННН НН C' Н н-с-с-с-с-н НН || Ш НННН H-C-C-C-C-N-H ННННН IVarrow_forward
- Rank the following compounds in order of decreasing dipole moment. |>||>||| ||>|||>| |>|||>|| |||>||>| O ||>>||| H F H F H c=c || H c=c F F IIIarrow_forwardchoose the description that best describes the geometry for the following charged species ch3-arrow_forwardWhy isn't the ketone in this compound converted to an acetal or hemiacetal by the alcohol and acid?arrow_forward
- What is the approximate bond angle around the nitrogen atom? HNH H Harrow_forwardOH 1. NaOCH2CH3 Q 2. CH3CH2Br (1 equiv) H3O+ Select to Draw 1. NaOCH2 CH3 2. CH3Br (1 equiv) heat Select to Edit Select to Drawarrow_forwardComplete and balance the following half-reaction in acidic solution. Be sure to include the proper phases for all species within the reaction. S₂O₃²⁻(aq) → S₄O₆²⁻(aq)arrow_forward
- Q Select to Edit NH3 (CH3)2CHCI (1 equiv) AICI 3 Select to Draw cat. H2SO4 SO3 (1 equiv) HO SOCl2 pyridine Select to Edit >arrow_forwardComplete and balance the following half-reaction in basic solution. Be sure to include the proper phases for all species within the reaction. Zn(s) → Zn(OH)₄²⁻(aq)arrow_forwardb. ὋΗ CH3CH2OH H2SO4arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License