
Concept explainers
Answer each question using the ball-and-stick model of compound A.
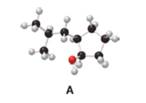
a. Give the IUPAC name for A, including
b. Classify A as a
c. Draw a stereoisomer for A and give its IUPAC name.
d. Draw a constitutional isomer that contains an
e. Draw a constitutional isomer that contains an ether and give its IUPAC name.
f. Draw the products formed (including stereochemistry) when A is treated with each reagent:
(a)
Interpretation: The IUPAC name for A, including
Concept introduction: One should follow the given steps to give the IUPAC name of cyclic alcohol. The first step is naming of ring that contains the carbon bonded to the hydroxyl group. While naming, the -e ending of parent cycloalkane must changed to the suffix -ol. The second step is numbering of carbon chain by providing lowest number to the
Answer to Problem 9.38P
The IUPAC name for A (including
Explanation of Solution
The given structure of alcohol is in the form of ball-and-stick model. It is converted into skeletal structure by replacing black ball with
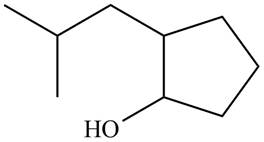
Figure 1
One should follow the given steps to give the IUPAC name of cyclic alcohol. The first step is naming of ring that contains the carbon bonded to the hydroxyl group. While naming, the -e ending of parent cycloalkane must changed to the suffix -ol. The second step is numbering of carbon chain by providing lowest number to the
The above structure of cyclic alcohol shows that the cyclic ring consists of
There are two stereogenic centers in the given structure (shown by
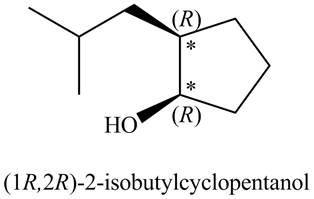
Figure 2
Thus, the IUPAC name for A (including
The IUPAC name for A (including
(b)
Interpretation: The given alcohol is to be classified as a
Concept introduction: Alcohols involve a hydroxyl group
Answer to Problem 9.38P
The given alcohol is classified as a
Explanation of Solution
Alcohols involve a hydroxyl group
In the given structure the carbon atom of alcohol (A) is bonded to two
The given alcohol is classified as a
(c)
Interpretation: A stereoisomer for ‘A’ is to be drawn, and its IUPAC name is to be stated.
Concept introduction: Isomers that differ to each other only in the way of orientation of atoms in space are called stereoisomers. They possess same functional group and same IUPAC name except for the prefixes (like cis, trans,
Answer to Problem 9.38P
A stereoisomer for ‘A’ (including its IUPAC name) is drawn in Figure 3.
Explanation of Solution
Isomers that differ to each other only in the way of orientation of atoms in space are called stereoisomers. They possess same functional group and same IUPAC name except for the prefixes (like cis, trans,
A stereoisomer for ‘A’ (including its IUPAC name) is drawn in Figure 3.
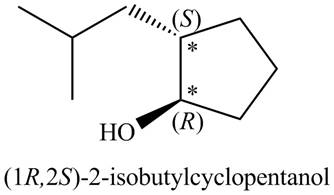
Figure 3
A stereoisomer for ‘A’ (including its IUPAC name) is drawn in Figure 3.
(d)
Interpretation: A constitutional isomer for A that contains an
Concept introduction: Isomers that differ in the way of connectivity of atoms to each other are called constitutional isomers. They may possess same or different functional groups. However, their IUPAC names are entirely different.
Answer to Problem 9.38P
A constitutional isomer for ‘A’ that contains an
Explanation of Solution
Isomers that differ in the way of connectivity of atoms to each other are called constitutional isomers. They may possess same or different functional groups. However, their IUPAC names are entirely different.
A constitutional isomer for ‘A’ that contains an
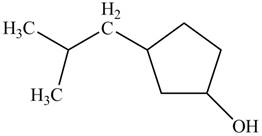
Figure 4
The above structure of cyclic alcohol shows that the cyclic ring consists of
A constitutional isomer for ‘A’ that contains an
(e)
Interpretation: A constitutional isomer for A that contains an ether is to be drawn, and its IUPAC name is to be stated.
Concept introduction: Isomers that differ in the way of connectivity of atoms to each other are called constitutional isomers. They may possess same or different functional groups. However, their IUPAC names are entirely different.
Answer to Problem 9.38P
A constitutional isomer for ‘A’ that contains an ether is drawn in Figure 5. Its IUPAC name is
Explanation of Solution
Isomers that differ in the way of connectivity of atoms to each other are called constitutional isomers. They may possess same or different functional groups. However, their IUPAC names are entirely different.
A constitutional isomer for ‘A’ that contains ether is drawn below.
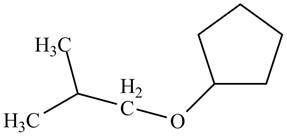
Figure 5
The above structure of cyclic alcohol shows that the cyclic ring consists of
A constitutional isomer for ‘A’ that contains an ether is drawn in Figure 5. Its IUPAC name is
(f)
Interpretation: The products (including stereochemistry) formed from the treatment of A with the each reagent are to be stated.
Concept introduction: In proton transfer reactions, the stereochemistry of the product is retained. The
Answer to Problem 9.38P
The products formed from the treatment of A with the reagent
Explanation of Solution
Products formed from reagent
The given reagent is
An alkoxide salt is required to prepare ether. The alkoxide salts are prepared from alcohols through the Bronsted-Lowry acid-base reaction. In this reaction,
The reaction involves only transfer of proton. Since the reaction does not involve cleavage of the
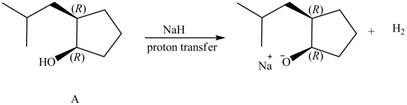
Figure 6
Products formed from reagent
The given reagent is
Alcohols undergo dehydration reaction in the presence of strong acids like
Thus, the products formed from the treatment of A with the given reagent are,
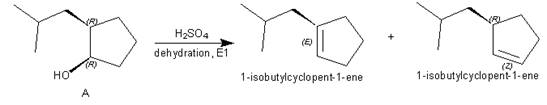
Figure 7
Mixture of products is obtained due to carbocation rearrangement, and having two
Products formed from reagent
The given reagent is
Alcohols undergo dehydration reaction in the presence of
Thus, the products formed from the treatment of A with the given reagent are,
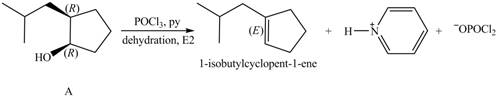
Figure 8
Products formed from reagent
The given reagent is
The reaction of alcohols with halogen acids
Since the given alcohol, A is
Thus, the products formed from the treatment of A with the given reagent are,
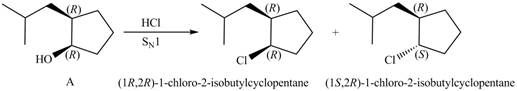
Figure 9
Products formed from reagent
The given reagent is
Alkyl chlorides are obtained by the reaction of
Since the given alcohol, A is
Thus, the products formed from the treatment of A with the given reagent are,
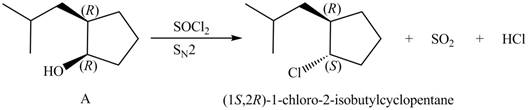
Figure 10
Products formed from reagent
The given reagent is
Alcohols are converted into alkyl tosylates by treatment with
Thus, the products formed from the treatment of A with the given reagent are,
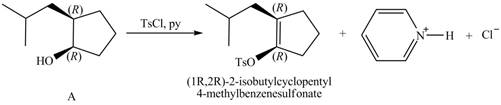
Figure 11
The products formed from the treatment of A with the reagent
Want to see more full solutions like this?
Chapter 9 Solutions
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
Additional Science Textbook Solutions
Biology: Life on Earth with Physiology (11th Edition)
Applications and Investigations in Earth Science (9th Edition)
Biology: Life on Earth (11th Edition)
Fundamentals Of Thermodynamics
Campbell Essential Biology with Physiology (5th Edition)
Human Biology: Concepts and Current Issues (8th Edition)
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
