
Organic Chemistry
11th Edition
ISBN: 9781118133576
Author: T. W. Graham Solomons, Craig Fryhle
Publisher: Wiley, John & Sons, Incorporated
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 9, Problem 5Q
How many 13C NMR signals would be given by the following compound?
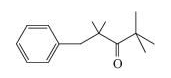
(a) 7
(b) 8
(c) 10
(d) 11
(e) 13
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Question 5: Name the following compound in two ways
using side chain and using prefix amine (Common name
and IUPAC name both)
CH3NH2
CH3CH2NHCH3
CH₂CH₂N(CH3)2
Draw the structure of diethyl methyl amine
Question 6. Write the balanced combustion reaction
for:
a. Hexane
b. Propyne
c. 2-pentene
Question 7: Write the following electrophilic
substitution reactions of benzene:
Hint: Use notes if you get confused
a. Halogenation reaction:
b. Nitration reaction :
c. Sulphonation reaction:
d. Alkylation reaction:
e. Aceylation reaction:
Question 4. Name the following structures
○
CH3-C-N-H
H
CH3CH2-C-N-H
H
CH3CH2-C-N-CH3
H
A. Add Water to below compound which 2-methyl 2-butene (addition Reaction)
H₂C
CH₂
CH,
+ H₂O-> ?
Major product?
Minor product?
B. Add Bromine to the compound which 2-methyl 2-butene (addition Reaction)
CH₂
CH₂
+ Br₂→ ?
Major product and Minor product both are same in this?
C. Add Hydrogen Bromide to the compound which 2-methyl 2-butene (addition
Reaction)
H,C
CH₂
CH₂
+ HBr
Major product?
Minor product?
D. Add Hydrogen to the compound which 2-methyl 2-butene (addition Reaction)
CH₂
CH₂
+ H₂
Major product and Minor product both are same in this?
Chapter 9 Solutions
Organic Chemistry
Ch. 9 - Prob. 1PPCh. 9 - PRACTICE PROBLEM
9.2 What compound with molecular...Ch. 9 - Prob. 3PPCh. 9 - PRACTICE PROBLEM 9.4 How many signals would each...Ch. 9 - Prob. 5PPCh. 9 - Prob. 6PPCh. 9 - Prob. 7PPCh. 9 - PRACTICE PROBLEM 9.7
The relative chemical shifts...Ch. 9 - Prob. 9PPCh. 9 - PRACTICE PROBLEM 9.9 Propose a structure for...
Ch. 9 - PRACTICE PROBLEM 9.10
What is the dihedral angle...Ch. 9 - PRACTICE PROBLEM 9.11 Draw the most stable chair...Ch. 9 - Prob. 13PPCh. 9 - Prob. 14PPCh. 9 - PRACTICE PROBLEM 9.13 How many signals would you...Ch. 9 - Prob. 16PPCh. 9 - Prob. 17PPCh. 9 - Prob. 18PPCh. 9 - Prob. 19PPCh. 9 - PRACTICE PROBLEM 9.18
What are the expected ratios...Ch. 9 - Given the mass spectrum in Figure 9.44 and the...Ch. 9 - Prob. 22PCh. 9 - 9.23 How many 13C NMR signals would you predict...Ch. 9 - Prob. 24PCh. 9 - 9.25 Propose structures for the compounds G and H...Ch. 9 - Prob. 26PCh. 9 - Prob. 27PCh. 9 - Compound Q has the molecular formula C7H8. The...Ch. 9 - 9.26 Explain in detail how you would distinguish...Ch. 9 - Prob. 30PCh. 9 - A compound with molecular formula C4H8O has a...Ch. 9 - In the mass spectrum of 2, 6-dimethyl-4-heptanol...Ch. 9 - Prob. 33PCh. 9 - What are the masses and structures of the ions...Ch. 9 - Prob. 35PCh. 9 - Ethyl bromide and methoxybenzene (shown below)...Ch. 9 - 9.34 The homologous series of primary amines, ,...Ch. 9 - Propose a structure that is consistent with each...Ch. 9 - 9.39 Propose structures for compounds E and F....Ch. 9 - Regarding compound J, C2HxCly, use the 1H NMR and...Ch. 9 - 9.38 When dissolved in , a compound (K) with the...Ch. 9 - Compound T (C5H8O) has a strong IR absorption band...Ch. 9 - Deduce the structure of the compound that gives...Ch. 9 - 9.45 Deduce the structure of the compound that...Ch. 9 - The 1H NMR spectrum of a solution of 1,...Ch. 9 - Acetic acid has a mass spectrum showing a...Ch. 9 - The 1H NMR peak for the hydroxyl proton of...Ch. 9 - The 1H NMR study of DMF (N, N-dimethylformamide)...Ch. 9 - 9.48 The mass spectra of many benzene derivatives...Ch. 9 - Prob. 52PCh. 9 - 1. Given the following information, elucidate the...Ch. 9 - Two compounds with the molecular formula C5H10O...Ch. 9 - Propose a structure that is consistent with each...Ch. 9 - 9.2 How many 1H NMR signals would the following...Ch. 9 - 9.3. How many 1H NMR signals would...Ch. 9 - 9.4 Which of these C6H14 isomers has the greatest...Ch. 9 - 9.5 How many 13C NMR signals would be given by the...Ch. 9 - Prob. 6QCh. 9 - 9.7 What is the structure of a compound C5H12...
Additional Science Textbook Solutions
Find more solutions based on key concepts
For parts a, b, and c, draw a diagram illustrating the alleleson homologous chromosomes for the following genot...
Genetic Analysis: An Integrated Approach (3rd Edition)
Classify each molecule as polar nonpolar. a. CS2 b. SO2 c. CH4 d. CH3CI
Introductory Chemistry (6th Edition)
A meteorite hits the upper atmosphere at 3000m/s , where the pressure is 0.1 atm and the temperature is 40C . H...
Fundamentals Of Thermodynamics
9. Why is the sphenoid bone called the keystone of the cranial floor?
Principles of Anatomy and Physiology
11. Birds and mammals are both endothermic, and both have four-chambered hearts. Most reptiles are ectothermic ...
Campbell Biology: Concepts & Connections (9th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 36) Complete the following multi-step reactions showing applications of enolate ions arising from ketones, esters, malonic ester, and keto ester, etc. (30 pts) (1) A NaOH, H₂O+ heat A NaOEt EtO OEt (11) EOH, H+ H. B LDA, H₂O+ -78°C B (i) NaOMe, Et-Br (ii) H₂O+, heat EtOOC (III) COOEt B A (i) NaOEt LiAlH 4-bromo-2-butene H₂O+ (ii) H3O+, heat Write the mechanism for Aldol Condensation (I A or B), and Claisen Condensation (II A).arrow_forward31) Complete two sets of reactions involving (R)-4-methyl-pent-2-ol producing racemic mixture of tertiary alcohols (D) and ketone derivative (C). Illustrate the mechanism of B and C or D. (25 pts) O OH 0 K2Cr2O7 Ph-CH2-Br, Mg, H2SO4 THF, H3O* (A) (D) Racemic mixture TsCl, Py (B) KCN, DMSO Ph-CH2-Br, Mg, THF, H3O+ (C) Mechanism for reactions B and C:arrow_forwardManoharan Mariappan, Ph.D., Dept. of Natur. Sci., NFC, Tallahassee, FL 33) Synthesize the aromatic compound containing para-substituted carbonyl compound starting from benzene. Illustrate the mechanism for reaction A. 1) NU (25 pts) A FeCl B (i) HNO3, H2SO4 (II) Sn, HCl(aq) NH₂ NO₂-D NH₂ (i) MeCO2Me, heat C (ii) K2Cr2O7/H2SO4 D (ii) SOCl2 (iii) 2 Et-NH2 Mechanism for reaction for the nitration of alkyl benzene (B-i): Characterize the product compound arising from the reaction D by IR and IH NMR spectral data: IR data (cm): 'H NMR data: Draw the structure and assign the chemical shift with the spin-splitting.arrow_forward
- Write structural formulas for the major products by doing addition reactions 1. You must add H2 as Pt is catalyst it does not take part in reactions only speed up the process H₂ CH2=CH-CH3 Pt 2. Add HCI break it into H and Cl CH3 HCI 3. Add Br2 only CC14 is catalyst CH3-CH=CH2 B12 CCl4 4. Add water to this and draw major product, H2SO4 is catalyst you have add water H20 in both the reaction below H₂SO4 CH3-CH=CH2 CH3 H2SO4/H₂O CH3-C=CH2 reflux ?arrow_forwardPlan the synthesis of the following compound using the starting material provided and any other reagents needed as long as carbon based reagents have 3 carbons or less. Either the retrosynthesis or the forward synthesis (mechanisms are not required but will be graded if provided) will be accepted if all necessary reagents and intermediates are shown (solvents and temperature requirements are not needed unless specifically involved in the reaction, i.e. DMSO in the Swern oxidation or heat in the KMnO4 oxidation). H Harrow_forwardHint These are benzene substitution reactions. ALCI3 and UV light are catalyst no part in reactions and triangle A means heating. A. Add ethyl for Et in benzene ring alkylation reaction EtCl = CH3CH2CL 1) EtC1 / AlCl3 / A ? B: Add Br to benzene ring ( substitution) 2) Br₂ / uv light ? C Add (CH3)2 CHCH2 in benzene ring ( substitution) (CH3)2CHCH,C1 / AICI, ?arrow_forward
- Draw the mechanism to make the alcohol 2-hexanol. Draw the Mechanism to make the alcohol 1-hexanol.arrow_forwardDraw the mechanism for the formation of diol by starting with 1-pentanal in... basic conditions then acidic conditions then draw the mechanism for the formation of a carboxylic acid from your product.arrow_forwardIdentify each chiral carbon as either R or S. Identify the overall carbohydrates as L or Darrow_forward
- Ethers can be formed via acid-catalyzed acetal formation. Draw the mechanism for the molecule below and ethanol.arrow_forwardHOCH, H HO CH-OH OH H OH 11 CH₂OH F II OH H H 0 + H OHarrow_forwardDraw the mechanism for the formation of diol by starting with one pen and all in... basic conditions then acidic conditions then draw the mechanism for the formation of a carboxylic acid from your product.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY