
Organic Chemistry
11th Edition
ISBN: 9781118133576
Author: T. W. Graham Solomons, Craig Fryhle
Publisher: Wiley, John & Sons, Incorporated
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 9, Problem 21PP
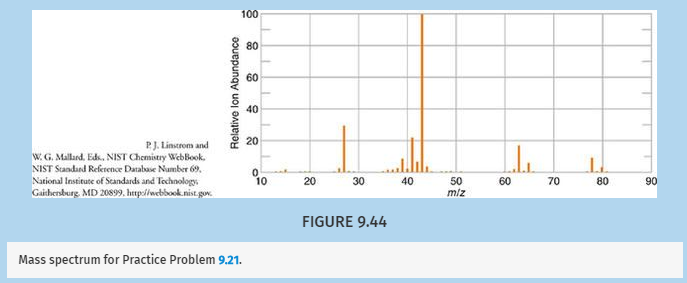
Given the mass spectrum in Figure 9.44 and the fact that the 1H NMR spectrum for this compound consists of only a large doublet and a small septet, what is the structure of the compound? Explain your reasoning.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
the decomposition of N2O5 is the first order with a half-life of 1.98 minutes. If the inital concentration of N2O5 is 0.200 M, what is the concentration after 6 minutes?a) 0.612 Mb) 0.035 Mc) 0.024 Md) 0.100 M
20.00 mL of 0.150 M HCI is titrated with 0.075 M NaOH. What volume of NaOH is needed?a) 50 mLb) 20 mLc) 40 mLd) 26.66 mL
20.00 mL of 0.150 M NaOH is titrated with 37.75 mL of HCI. What is the molarity of the HCI?a) 0.150 Mb) 0.079 Mc) 0.025 Md) 0.050 M
Chapter 9 Solutions
Organic Chemistry
Ch. 9 - Prob. 1PPCh. 9 - PRACTICE PROBLEM
9.2 What compound with molecular...Ch. 9 - Prob. 3PPCh. 9 - PRACTICE PROBLEM 9.4 How many signals would each...Ch. 9 - Prob. 5PPCh. 9 - Prob. 6PPCh. 9 - Prob. 7PPCh. 9 - PRACTICE PROBLEM 9.7
The relative chemical shifts...Ch. 9 - Prob. 9PPCh. 9 - PRACTICE PROBLEM 9.9 Propose a structure for...
Ch. 9 - PRACTICE PROBLEM 9.10
What is the dihedral angle...Ch. 9 - PRACTICE PROBLEM 9.11 Draw the most stable chair...Ch. 9 - Prob. 13PPCh. 9 - Prob. 14PPCh. 9 - PRACTICE PROBLEM 9.13 How many signals would you...Ch. 9 - Prob. 16PPCh. 9 - Prob. 17PPCh. 9 - Prob. 18PPCh. 9 - Prob. 19PPCh. 9 - PRACTICE PROBLEM 9.18
What are the expected ratios...Ch. 9 - Given the mass spectrum in Figure 9.44 and the...Ch. 9 - Prob. 22PCh. 9 - 9.23 How many 13C NMR signals would you predict...Ch. 9 - Prob. 24PCh. 9 - 9.25 Propose structures for the compounds G and H...Ch. 9 - Prob. 26PCh. 9 - Prob. 27PCh. 9 - Compound Q has the molecular formula C7H8. The...Ch. 9 - 9.26 Explain in detail how you would distinguish...Ch. 9 - Prob. 30PCh. 9 - A compound with molecular formula C4H8O has a...Ch. 9 - In the mass spectrum of 2, 6-dimethyl-4-heptanol...Ch. 9 - Prob. 33PCh. 9 - What are the masses and structures of the ions...Ch. 9 - Prob. 35PCh. 9 - Ethyl bromide and methoxybenzene (shown below)...Ch. 9 - 9.34 The homologous series of primary amines, ,...Ch. 9 - Propose a structure that is consistent with each...Ch. 9 - 9.39 Propose structures for compounds E and F....Ch. 9 - Regarding compound J, C2HxCly, use the 1H NMR and...Ch. 9 - 9.38 When dissolved in , a compound (K) with the...Ch. 9 - Compound T (C5H8O) has a strong IR absorption band...Ch. 9 - Deduce the structure of the compound that gives...Ch. 9 - 9.45 Deduce the structure of the compound that...Ch. 9 - The 1H NMR spectrum of a solution of 1,...Ch. 9 - Acetic acid has a mass spectrum showing a...Ch. 9 - The 1H NMR peak for the hydroxyl proton of...Ch. 9 - The 1H NMR study of DMF (N, N-dimethylformamide)...Ch. 9 - 9.48 The mass spectra of many benzene derivatives...Ch. 9 - Prob. 52PCh. 9 - 1. Given the following information, elucidate the...Ch. 9 - Two compounds with the molecular formula C5H10O...Ch. 9 - Propose a structure that is consistent with each...Ch. 9 - 9.2 How many 1H NMR signals would the following...Ch. 9 - 9.3. How many 1H NMR signals would...Ch. 9 - 9.4 Which of these C6H14 isomers has the greatest...Ch. 9 - 9.5 How many 13C NMR signals would be given by the...Ch. 9 - Prob. 6QCh. 9 - 9.7 What is the structure of a compound C5H12...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Police Captain Jeffers has suffered a myocardial infarction. a. Explain to his (nonmedically oriented) family w...
Human Physiology: An Integrated Approach (8th Edition)
Define histology.
Fundamentals of Anatomy & Physiology (11th Edition)
1. What is thermochemistry? Why is it important?
Chemistry: Structure and Properties (2nd Edition)
Fibrous connective tissue consists of ground substance and fibers that provide strength, support, and flexibili...
Human Biology: Concepts and Current Issues (8th Edition)
If someone at the other end of a room smokes a cigarette, you may breathe in some smoke. The movement of smoke ...
Campbell Essential Biology with Physiology (5th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- in the following reaction, the OH- acts as which of these?NO2- (aq) + H2O (l) ⇌ OH- (aq) + HNO2 (aq)a) not a weak acidb) basec) acidarrow_forwardfind the pH of a buffer made from 0.20 M HNO2 and 0.10 M NaNO2. Ka= 4.0 x 10-4a) 4.00b) 3.40c) 3.70d) 3.10arrow_forwardthe Ka for sodium dihydrogen phosphate is 6.32 x 10-8. Find the pH of a buffer made from 0.15 M H2PO4- and 0.15 M HPO42-.a) 6.98b) 7.42c) 7.00d) 7.20arrow_forward
- Find the equilibrium concentration of H3O+ starting with 0.072 M solution of acetic acid. Ka = 1.8 x 10-5. Acetic acid is HC2H3O2 (aq).HC2H3O2 (aq) + H2O (l) ⇌ H3O (aq) + C2H3O2- (aq) a) 1.3 x 10-6 b) 1.1 x 10-3 c) 1.5 x 10-2 d) 3.6 x 10-5arrow_forwardin VSEPR Theory, AX2 isarrow_forwardcalculate the pH of 0.066 M Ca(OH)2. Remember stoichiometry.arrow_forward
- Find the equilibrium concentration of H3O+ starting with 0.072 M solution of acetic acid. Ka = 1.8 x 10-5. Acetic acid is HC2H3O2 (aq).HC2H3O2 (aq) + H2O (l) ⇌ H3O (aq) + C2H3O2- (aq)arrow_forwardin VSEPR Theory AX2 isa) tetrahedralb) octahedralc) lineard) trigonal bipyramidarrow_forwardCheck How many signals would you expect to find in the H NMR spectrum of each given compound? Part 1 of 2 Part 2 of 2 Br Br 2. Cl X 2 © 2025 McGraw Hill LLC. All Rights Resarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY