
Organic Chemistry
11th Edition
ISBN: 9781118133576
Author: T. W. Graham Solomons, Craig Fryhle
Publisher: Wiley, John & Sons, Incorporated
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 9, Problem 39P
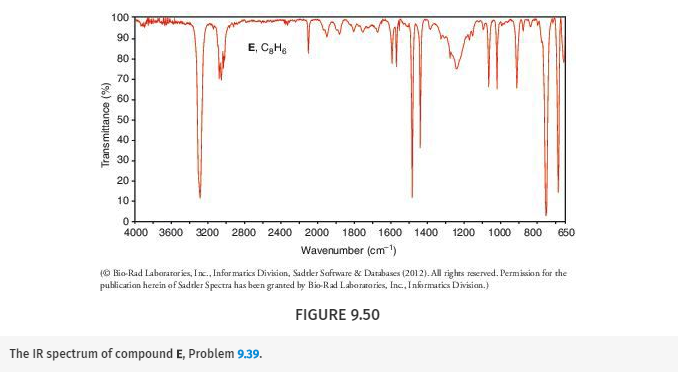
Propose structures for compounds E and F. Compound E (C8H6) reacts with 2 molar equivalents of bromine to form F (C8H6Br4). E has the IR spectrum shown in Fig. 9.50. What are the structures of E and F?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw the mechanism for the acid-catalyzed dehydration of 2-methyl-hexan-2-ol.
Draw the product of the reaction no mechanism required.
Identify the glycosidic linkage.
Chapter 9 Solutions
Organic Chemistry
Ch. 9 - Prob. 1PPCh. 9 - PRACTICE PROBLEM
9.2 What compound with molecular...Ch. 9 - Prob. 3PPCh. 9 - PRACTICE PROBLEM 9.4 How many signals would each...Ch. 9 - Prob. 5PPCh. 9 - Prob. 6PPCh. 9 - Prob. 7PPCh. 9 - PRACTICE PROBLEM 9.7
The relative chemical shifts...Ch. 9 - Prob. 9PPCh. 9 - PRACTICE PROBLEM 9.9 Propose a structure for...
Ch. 9 - PRACTICE PROBLEM 9.10
What is the dihedral angle...Ch. 9 - PRACTICE PROBLEM 9.11 Draw the most stable chair...Ch. 9 - Prob. 13PPCh. 9 - Prob. 14PPCh. 9 - PRACTICE PROBLEM 9.13 How many signals would you...Ch. 9 - Prob. 16PPCh. 9 - Prob. 17PPCh. 9 - Prob. 18PPCh. 9 - Prob. 19PPCh. 9 - PRACTICE PROBLEM 9.18
What are the expected ratios...Ch. 9 - Given the mass spectrum in Figure 9.44 and the...Ch. 9 - Prob. 22PCh. 9 - 9.23 How many 13C NMR signals would you predict...Ch. 9 - Prob. 24PCh. 9 - 9.25 Propose structures for the compounds G and H...Ch. 9 - Prob. 26PCh. 9 - Prob. 27PCh. 9 - Compound Q has the molecular formula C7H8. The...Ch. 9 - 9.26 Explain in detail how you would distinguish...Ch. 9 - Prob. 30PCh. 9 - A compound with molecular formula C4H8O has a...Ch. 9 - In the mass spectrum of 2, 6-dimethyl-4-heptanol...Ch. 9 - Prob. 33PCh. 9 - What are the masses and structures of the ions...Ch. 9 - Prob. 35PCh. 9 - Ethyl bromide and methoxybenzene (shown below)...Ch. 9 - 9.34 The homologous series of primary amines, ,...Ch. 9 - Propose a structure that is consistent with each...Ch. 9 - 9.39 Propose structures for compounds E and F....Ch. 9 - Regarding compound J, C2HxCly, use the 1H NMR and...Ch. 9 - 9.38 When dissolved in , a compound (K) with the...Ch. 9 - Compound T (C5H8O) has a strong IR absorption band...Ch. 9 - Deduce the structure of the compound that gives...Ch. 9 - 9.45 Deduce the structure of the compound that...Ch. 9 - The 1H NMR spectrum of a solution of 1,...Ch. 9 - Acetic acid has a mass spectrum showing a...Ch. 9 - The 1H NMR peak for the hydroxyl proton of...Ch. 9 - The 1H NMR study of DMF (N, N-dimethylformamide)...Ch. 9 - 9.48 The mass spectra of many benzene derivatives...Ch. 9 - Prob. 52PCh. 9 - 1. Given the following information, elucidate the...Ch. 9 - Two compounds with the molecular formula C5H10O...Ch. 9 - Propose a structure that is consistent with each...Ch. 9 - 9.2 How many 1H NMR signals would the following...Ch. 9 - 9.3. How many 1H NMR signals would...Ch. 9 - 9.4 Which of these C6H14 isomers has the greatest...Ch. 9 - 9.5 How many 13C NMR signals would be given by the...Ch. 9 - Prob. 6QCh. 9 - 9.7 What is the structure of a compound C5H12...
Additional Science Textbook Solutions
Find more solutions based on key concepts
4. What five specific threats to biodiversity are described in this chapter? Provide an example of each.
Biology: Life on Earth (11th Edition)
The method of measuring humidity must be explained. Concept introduction: Amount of water vapor in air is diffe...
Living By Chemistry: First Edition Textbook
If a compound has a molecular ion with an odd-numbered mass, then the compound contains an odd number of nitrog...
Organic Chemistry (8th Edition)
What are the features of the cells, ground substance, and fibers that make up connective tissue?
Principles of Anatomy and Physiology
The plane extending through each block diagram is called the__________
Applications and Investigations in Earth Science (9th Edition)
The following results were obtained from a broth dilution test for microbial susceptibility. Antibiotic Concent...
Microbiology: An Introduction
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the mechanism for the substitution reaction converting an alcohol into an alkyl halide. If chirality is important to the reaction include it.arrow_forwardWrite, in words three different reactions we can use to make an alcohol.arrow_forwardDraw the reduction mechanism for the reduction of the aldehyde.arrow_forward
- What is the product of the reaction of XeF4 with H2O? Group of answer choices H2XeF2 H2XeF4 XeO3 H2XeOarrow_forwardWhile noble gas exerts the strongest London (dispersion) forces on neighboring atoms? Group of answer choices Xe Ar Kr Nearrow_forwardWhich of the following elements is corrosive to your skin due to that element breaking down C=C bonds? Group of answer choices fluorine iodine bromine chlorinearrow_forward
- What the best source of sulfide to use on a small scale in the lab? Group of answer choices thiourea H2S NaHS Na2Sarrow_forwardWhich of the following statements about sulfur is FALSE? Group of answer choices H2S is the product of an oxygen-depleted ecosystem. In the acid mine drainage reaction, FeS2 is a product. One allotrope of sulfur has the formula S20. In the environment, bacterial oxidation can convert S2− to elemental S or SO42−.arrow_forwardOf the following choices, which is the best reason that most materials DON'T spontaneously combust even though our atmosphere is about 21% oxygen? Group of answer choices The reduction of O2 in the gas phase (O2 + e− → O2−) is spontaneous. The reduction of O2 in acid solution (O2 + H+ + e− → HO2(aq)) is spontaneous. O2 is not a reactant in combustion. The O2 bond dissociation energy is 494 kJ/mol, leading to a high activation energy for combustion.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY