
Chemistry
3rd Edition
ISBN: 9780073402734
Author: Julia Burdge
Publisher: MCGRAW-HILL HIGHER EDUCATION
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 8.1, Problem 1PPC
Practice Problem CONCEPTUALIZE
CONCEPTUALIZE
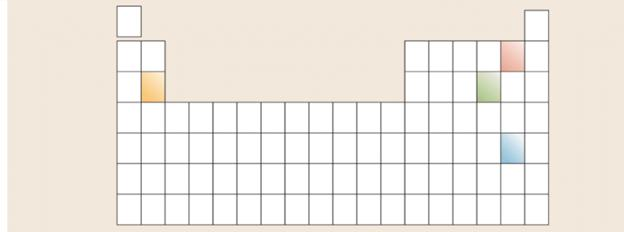
For each of the highlighted positions on the periodic table, write a Lewis structure for an atom of the element and for the common ion that it forms. Use the generic symbol X for each element. For example, rather than writing
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Are there any alternative methods better than the MOHR titration to quantitatively determine salt in a sample?
hybridization of nitrogen of complex molecules
Using reaction free energy to predict equilibrium composition
Consider the following equilibrium:
2NO2 (g) = N2O4(g)
AGº = -5.4 kJ
Now suppose a reaction vessel is filled with 4.53 atm of dinitrogen tetroxide (N2O4) at 279. °C. Answer the following questions about this system:
Under these conditions, will the pressure of N2O4 tend to rise or fall?
Is it possible to reverse this tendency by adding NO2?
In other words, if you said the pressure of N2O4 will tend to rise, can that
be changed to a tendency to fall by adding NO2? Similarly, if you said the
pressure of N2O4 will tend to fall, can that be changed to a tendency to
'2'
rise by adding NO2?
If you said the tendency can be reversed in the second question, calculate
the minimum pressure of NO 2 needed to reverse it.
Round your answer to 2 significant digits.
00
rise
☐ x10
fall
yes
no
☐ atm
G
Ar
1
Chapter 8 Solutions
Chemistry
Ch. 8.1 - Practice ProblemATTEMPT Write Lewis dot symbols...Ch. 8.1 - Practice Problem BUILD
Indicate the charge on...Ch. 8.1 - Practice ProblemCONCEPTUALIZE For each of the...Ch. 8.1 - 8.1.1 Using only a periodic table, determine the...Ch. 8.1 - 8.1.2 Using only a periodic table, determine the...Ch. 8.1 - To which group does element X belong if its Lewis...Ch. 8.1 - Prob. 4CPCh. 8.2 - Prob. 1PPACh. 8.2 - Practice ProblemBUILD Arrange the compounds NaF,...Ch. 8.2 - Practice ProblemCONCEPTUALIZE Common ions of four...
Ch. 8.2 - 8.2.1 Will the lattice energy of KF be larger or...Ch. 8.2 - 8.2.2 Using the following data, calculate the...Ch. 8.2 - 8.2.3 Lattice energies are graphed for three...Ch. 8.3 - Practice ProblemATTEMPT Using data from Figures...Ch. 8.3 - Prob. 1PPBCh. 8.3 - Prob. 1PPCCh. 8.4 - Practice Problem ATTEMPT Classify the following...Ch. 8.4 - Prob. 1PPBCh. 8.4 - Prob. 1PPCCh. 8.4 - In which of the following molecules are the bonds...Ch. 8.4 - Using data from Table 8.5, calculate the magnitude...Ch. 8.4 - Prob. 3CPCh. 8.4 - Prob. 4CPCh. 8.5 - Prob. 1PPACh. 8.5 - Prob. 1PPBCh. 8.5 - Two pairs of elements are highlighted in the...Ch. 8.5 - Identify the correct Lewis structure for formic...Ch. 8.5 - Identity the correct Lewis structure for hydrogen...Ch. 8.6 - Prob. 1PPACh. 8.6 - Prob. 1PPBCh. 8.6 - Prob. 1PPCCh. 8.6 - Determine the formal charges on H, C, and N,...Ch. 8.6 - 8.6.2 Which of the Lewis structures shown is most...Ch. 8.7 - Prob. 1PPACh. 8.7 - Practice ProblemBUILD Draw the Lewis structure for...Ch. 8.7 - Practice Problem CONCEPTUALIZE
Of the three Lewis...Ch. 8.7 - Indicate which of the following are resonance...Ch. 8.7 - 8.7.2 How many resonance structures can be drawn...Ch. 8.8 - Prob. 1PPACh. 8.8 - Prob. 1PPBCh. 8.8 - Practice Problem CONCEPTUALIZE
The hypothetical...Ch. 8.8 - In which of the following species does the central...Ch. 8.8 - Prob. 2CPCh. 8.8 - In which species does the central atom obey the...Ch. 8.8 - 8.8.4 How many lone pairs are there on the central...Ch. 8.9 - Prob. 1PPACh. 8.9 - Practice ProblemBUILD Use Lewis structures and...Ch. 8.9 - Prob. 1PPCCh. 8.9 - 8.9.1 Use data from Table 8.6 to estimate for the...Ch. 8.9 - Use data from Table 8.6 to estimate Δ H rxn for...Ch. 8.9 - Use bond enthalpies to determine Δ H rxn for the...Ch. 8.9 - Prob. 4CPCh. 8.10 - Practice ProblemATTEMPT Draw all possible...Ch. 8.10 - Prob. 1PPBCh. 8.10 - Practice ProblemCONCEPTUALIZE The Lewis structure...Ch. 8.11 - Prob. 1PPACh. 8.11 - Prob. 1PPBCh. 8.11 - Prob. 1PPCCh. 8.12 - Prob. 1PPACh. 8.12 - Prob. 1PPBCh. 8.12 - Prob. 1PPCCh. 8.13 - Prob. 1PPACh. 8.13 - Practice Problem BUILD
Using the following...Ch. 8.13 - Prob. 1PPCCh. 8 - 8.1
Which of the following atoms must always obey...Ch. 8 - Prob. 2KSPCh. 8 - Prob. 3KSPCh. 8 - Prob. 4KSPCh. 8 - What is a Lewis dot symbol? What elements do we...Ch. 8 - Use the second member of each group from Group 1A...Ch. 8 - Prob. 3QPCh. 8 - 8.4 Write Lewis dot symbols for the following...Ch. 8 - Write Lewis dot symbols for the following atoms...Ch. 8 - Prob. 6QPCh. 8 - Prob. 7QPCh. 8 - Name five metals and five nonmetals that are very...Ch. 8 - Prob. 9QPCh. 8 - Prob. 10QPCh. 8 - Prob. 11QPCh. 8 - The term molar mass was introduced in Chapter 3....Ch. 8 - Prob. 13QPCh. 8 - Prob. 14QPCh. 8 - Prob. 15QPCh. 8 - Explain how the lattice energy of an ionic...Ch. 8 - Prob. 17QPCh. 8 - Prob. 18QPCh. 8 - 8.19 Use the Born-Haber cycle outlined in Section...Ch. 8 - Calculate the lattice energy of CaCl 2 . Use data...Ch. 8 - An ionic bond is formed between a cation A + and...Ch. 8 - Prob. 22QPCh. 8 - Use Lewis dot symbols to show the transfer of...Ch. 8 - Write the Lewis dot symbols of the reactants and...Ch. 8 - 8.25 Describe Lewis’s contribution to our...Ch. 8 - Prob. 26QPCh. 8 - Prob. 27QPCh. 8 - Prob. 28QPCh. 8 - Prob. 29QPCh. 8 - Prob. 30QPCh. 8 - Prob. 31QPCh. 8 - Prob. 32QPCh. 8 - Prob. 33QPCh. 8 - Define electronegativity, and explain the...Ch. 8 - Prob. 35QPCh. 8 - Prob. 36QPCh. 8 - List the following bonds in order of increasing...Ch. 8 - Classify the following bonds as covalent, polar...Ch. 8 - 8.41 Classify the following bonds as covalent,...Ch. 8 - 8.42 List the following bonds in order of...Ch. 8 - Prob. 41QPCh. 8 - Prob. 42QPCh. 8 - Draw Lewis structures for the following molecules...Ch. 8 - Draw Lewis structures for the following molecules:...Ch. 8 - Prob. 45QPCh. 8 - Prob. 46QPCh. 8 - 8.51 Draw Lewis structures for the following ions:...Ch. 8 - Draw Lewis structures for the following ions: (a)...Ch. 8 - Prob. 49QPCh. 8 - Prob. 50QPCh. 8 - Prob. 51QPCh. 8 - Prob. 52QPCh. 8 - Prob. 53QPCh. 8 - 8.58 Draw three resonance structures for the...Ch. 8 - Prob. 55QPCh. 8 - Prob. 56QPCh. 8 - Draw three reasonable resonance structures for the...Ch. 8 - Draw three resonance structures for the molecule N...Ch. 8 - Prob. 59QPCh. 8 - Prob. 60QPCh. 8 - Prob. 61QPCh. 8 - Prob. 62QPCh. 8 - Prob. 63QPCh. 8 - Prob. 64QPCh. 8 - Prob. 65QPCh. 8 - The AlI 3 molecule has an incomplete octet around...Ch. 8 - Prob. 67QPCh. 8 - Prob. 68QPCh. 8 - 8.73 Write a Lewis structure for Does this...Ch. 8 - Prob. 70QPCh. 8 - Prob. 71QPCh. 8 - 8.76 Draw two resonance structures for the bromate...Ch. 8 - Prob. 73QPCh. 8 - What is bond enthalpy? Bond enthalpies of...Ch. 8 - Prob. 75QPCh. 8 - Prob. 76QPCh. 8 - Prob. 77QPCh. 8 - Prob. 78QPCh. 8 - For the reaction 2 C 2 H 6 ( g ) + 7 O 2 ( g ) → 4...Ch. 8 - Prob. 80QPCh. 8 - 8.85. Use average bond enthalpies from Table 8.6...Ch. 8 - Prob. 82APCh. 8 - Prob. 83APCh. 8 - Prob. 84APCh. 8 - Prob. 85APCh. 8 - Prob. 86APCh. 8 - 8.91 Describe some characteristics of an ionic...Ch. 8 - Prob. 88APCh. 8 - Prob. 89APCh. 8 - Prob. 90APCh. 8 - Prob. 91APCh. 8 - Prob. 92APCh. 8 - Prob. 93APCh. 8 - Prob. 94APCh. 8 - Prob. 95APCh. 8 - Prob. 96APCh. 8 - Prob. 97APCh. 8 - Prob. 98APCh. 8 - Prob. 99APCh. 8 - Prob. 100APCh. 8 - Which of the following species are isoelectronic:...Ch. 8 - Prob. 102APCh. 8 - 8.107 Draw two resonance structures for each...Ch. 8 - The following species have been detected in...Ch. 8 - The amide ion ( NH 2 − ) is a Brø�nsted base. Use...Ch. 8 - Prob. 106APCh. 8 - The triiodide ion ( I 3 − ) in which the I atoms...Ch. 8 - Prob. 108QPCh. 8 - In 1999, an unusual cation containing only...Ch. 8 - Prob. 110QPCh. 8 - Prob. 111QPCh. 8 - Prob. 112APCh. 8 - In the gas phase, aluminum chloride exists as a...Ch. 8 - Prob. 114APCh. 8 - Calculate Δ H º for the reaction H 2 ( g ) + I 2 (...Ch. 8 - Draw Lewis structures for the following organic...Ch. 8 - Prob. 117APCh. 8 - Prob. 118APCh. 8 - Prob. 119APCh. 8 - Write three resonance structures for (a) the...Ch. 8 - Prob. 121APCh. 8 - Prob. 122APCh. 8 - Prob. 123APCh. 8 - Prob. 124APCh. 8 - Prob. 125APCh. 8 - Prob. 126APCh. 8 - Prob. 127APCh. 8 - Among the common inhaled anesthetics are:...Ch. 8 - Prob. 129APCh. 8 - Prob. 130APCh. 8 - Prob. 131APCh. 8 - 8.136 Using this and data from Appendix 2,...Ch. 8 - Prob. 133QPCh. 8 - Prob. 134QPCh. 8 - Prob. 135QPCh. 8 - Prob. 136QPCh. 8 - Prob. 137QPCh. 8 - Prob. 138APCh. 8 - Prob. 139APCh. 8 - Although nitrogen dioxide ( NO 2 ) is a stable...Ch. 8 - 8.145 The chlorine nitrate molecule is believed...Ch. 8 - The hydroxyl radical ( OH ) plays an important...Ch. 8 - Prob. 143APCh. 8 - Prob. 144APCh. 8 - Prob. 1SEPPCh. 8 - 2. Use formal charges to choose the best of the...Ch. 8 - Prob. 3SEPPCh. 8 - Prob. 4SEPP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Why do we analyse salt?arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. H H CH3OH, H+ H Select to Add Arrows H° 0:0 'H + Q HH ■ Select to Add Arrows CH3OH, H* H. H CH3OH, H+ HH ■ Select to Add Arrows i Please select a drawing or reagent from the question areaarrow_forwardWhat are examples of analytical methods that can be used to analyse salt in tomato sauce?arrow_forward
- A common alkene starting material is shown below. Predict the major product for each reaction. Use a dash or wedge bond to indicate the relative stereochemistry of substituents on asymmetric centers, where applicable. Ignore any inorganic byproducts H Šali OH H OH Select to Edit Select to Draw 1. BH3-THF 1. Hg(OAc)2, H2O =U= 2. H2O2, NaOH 2. NaBH4, NaOH + Please select a drawing or reagent from the question areaarrow_forwardWhat is the MOHR titration & AOAC method? What is it and how does it work? How can it be used to quantify salt in a sample?arrow_forwardPredict the major products of this reaction. Cl₂ hv ? Draw only the major product or products in the drawing area below. If there's more than one major product, you can draw them in any arrangement you like. Be sure you use wedge and dash bonds if necessary, for example to distinguish between major products with different stereochemistry. If there will be no products because there will be no significant reaction, just check the box under the drawing area and leave it blank. Note for advanced students: you can ignore any products of repeated addition. Explanation Check Click and drag to start drawing a structure. 80 10 m 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility DII A F1 F2 F3 F4 F5 F6 F7 F8 EO F11arrow_forward
- Given a system with an anodic overpotential, the variation of η as a function of current density- at low fields is linear.- at higher fields, it follows Tafel's law.Calculate the range of current densities for which the overpotential has the same value when calculated for both cases (the maximum relative difference will be 5%, compared to the behavior for higher fields).arrow_forwardUsing reaction free energy to predict equilibrium composition Consider the following equilibrium: N2 (g) + 3H2 (g) = 2NH3 (g) AGº = -34. KJ Now suppose a reaction vessel is filled with 8.06 atm of nitrogen (N2) and 2.58 atm of ammonia (NH3) at 106. °C. Answer the following questions about this system: rise Under these conditions, will the pressure of N2 tend to rise or fall? ☐ x10 fall Is it possible to reverse this tendency by adding H₂? In other words, if you said the pressure of N2 will tend to rise, can that be changed to a tendency to fall by adding H2? Similarly, if you said the pressure of N will tend to fall, can that be changed to a tendency to rise by adding H₂? If you said the tendency can be reversed in the second question, calculate the minimum pressure of H₂ needed to reverse it. Round your answer to 2 significant digits. yes no ☐ atm Х ด ? olo 18 Ararrow_forwardFour liters of an aqueous solution containing 6.98 mg of acetic acid were prepared. At 25°C, the measured conductivity was 5.89x10-3 mS cm-1. Calculate the degree of dissociation of the acid and its ionization constant.Molecular weights: O (15.999), C (12.011), H (1.008).Limiting molar ionic conductivities (λ+0 and λ-0) of Ac-(aq) and H+(aq): 40.9 and 349.8 S cm-2 mol-1.arrow_forward
- Determine the change in Gibbs energy, entropy, and enthalpy at 25°C for the battery from which the data in the table were obtained.T (°C) 15 20 25 30 35Eo (mV) 227.13 224.38 221.87 219.37 216.59Data: n = 1, F = 96485 C mol–1arrow_forwardIndicate the correct options.1. The units of the transport number are Siemens per mole.2. The Siemens and the ohm are not equivalent.3. The Van't Hoff factor is dimensionless.4. Molar conductivity does not depend on the electrolyte concentration.arrow_forwardIdeally nonpolarizable electrodes can1. participate as reducers in reactions.2. be formed only with hydrogen.3. participate as oxidizers in reactions.4. form open and closed electrochemical systems.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
Atomic Number, Atomic Mass, and the Atomic Structure | How to Pass ChemistryThe Nucleus: Crash Course Chemistry #1; Author: Crash Course;https://www.youtube.com/watch?v=FSyAehMdpyI;License: Standard YouTube License, CC-BY