
Concept explainers
(a)
Interpretation:
Balanced equation for the reaction between
Concept Introduction:
Chemical equation is the representation of a
According to physical and chemical properties, the elements can be further divided into metals, non-metals and metalloids.
In a group, the metallic character of an element increases from top to bottom whereas in a period, it decreases from left to right.
The classification elements in the periodic table as metals, nonmetals, or metalloids can be given as
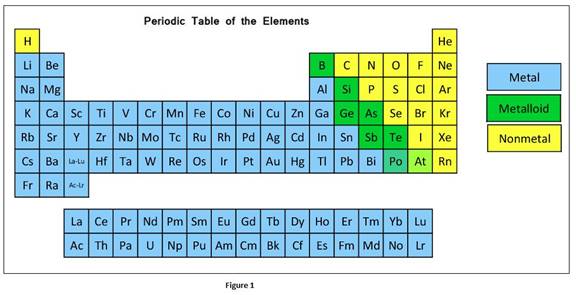
Oxide is the compound formed when oxygen reacts with another element. Oxides formed with metals are basic
Most of oxides formed with nonmetals are acidic.
Amphoteric oxides have the properties of bases and acid. Elements that are in the intermediate position of periodic table form amphoteric oxide.
(b)
Interpretation:
Balanced equation for the reaction between
Concept Introduction:
Chemical equation is the representation of a chemical reaction, in which the reactants and products of the reactions are represented left and right side of an arrow respectively by using their respective chemical formulas.
According to physical and chemical properties, the elements can be further divided into metals, non-metals and metalloids.
In a group, the metallic character of an element increases from top to bottom whereas in a period, it decreases from left to right.
The classification elements in the periodic table as metals, nonmetals, or metalloids can be given as
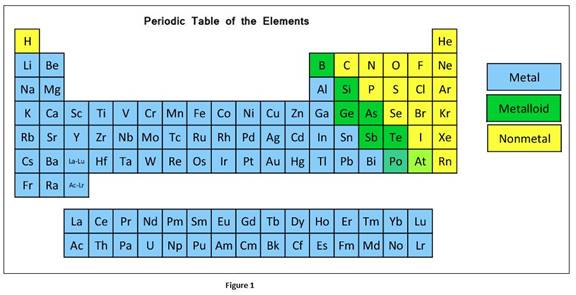
Oxide is the compound formed when oxygen reacts with another element. Oxides formed with metals are basic
Most of oxides formed with nonmetals are acidic.
Amphoteric oxides have the properties of bases and acid. Elements that are in the intermediate position of periodic table form amphoteric oxide.
(c)
Interpretation:
Balanced equation for the reaction between
Concept Introduction:
Chemical equation is the representation of a chemical reaction, in which the reactants and products of the reactions are represented left and right side of an arrow respectively by using their respective chemical formulas.
According to physical and chemical properties, the elements can be further divided into metals, non-metals and metalloids.
In a group, the metallic character of an element increases from top to bottom whereas in a period, it decreases from left to right.
The classification elements in the periodic table as metals, nonmetals, or metalloids can be given as
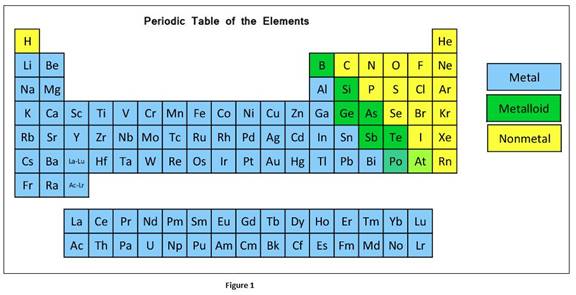
Oxide is the compound formed when oxygen reacts with another element. Oxides formed with metals are basic
Most of oxides formed with nonmetals are acidic.
Amphoteric oxides have the properties of bases and acid. Elements that are in the intermediate position of periodic table form amphoteric oxide.
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
EBK GENERAL CHEMISTRY: THE ESSENTIAL CO
- Draw everything on a piece of paper including every single step and each name provided using carbons less than 3 please.arrow_forwardTopics] [References] Write an acceptable IUPAC name for the compound below. (Only systematic names, not common names are accepted by this question.) Keep the information page open for feedback reference. H The IUPAC name isarrow_forward[Review Topics] [References] Write an acceptable IUPAC name for the compound below. (Only systematic names, not common names are accepted by this question.) Keep the information page open for feedback reference. The IUPAC name is Submit Answer Retry Entire Group 9 more group attempts remainingarrow_forward
- Please draw.arrow_forwardA chromatogram with ideal Gaussian bands has tR = 9.0 minutes and w1/2 = 2.0 minutes. Find the number of theoretical plates that are present, and calculate the height of each theoretical plate if the column is 10 centimeters long.arrow_forwardAn open tubular column has an inner diameter of 207 micrometers, and the thickness of the stationary phase on the inner wall is 0.50 micrometers. Unretained solute passes through in 63 seconds and a particular solute emerges at 433 seconds. Find the distribution constant for this solute and find the fraction of time spent in the stationary phase.arrow_forward
- Consider a chromatography column in which Vs= Vm/5. Find the retention factor if Kd= 3 and Kd= 30.arrow_forwardTo improve chromatographic separation, you must: Increase the number of theoretical plates on the column. Increase the height of theoretical plates on the column. Increase both the number and height of theoretical plates on the column. Increasing the flow rate of the mobile phase would Increase longitudinal diffusion Increase broadening due to mass transfer Increase broadening due to multiple paths You can improve the separation of components in gas chromatography by: Rasing the temperature of the injection port Rasing the temperature of the column isothermally Rasing the temperature of the column using temperature programming In GC, separation between two different solutes occurs because the solutes have different solubilities in the mobile phase the solutes volatilize at different rates in the injector the solutes spend different amounts of time in the stationary phasearrow_forwardplease draw and example of the following: Show the base pair connection(hydrogen bond) in DNA and RNAarrow_forward
- Naming and drawing secondary Write the systematic (IUPAC) name for each of the following organic molecules: CH3 Z structure CH3 CH2 CH2 N-CH3 CH3-CH2-CH2-CH-CH3 NH CH3-CH-CH2-CH2-CH2-CH2-CH2-CH3 Explanation Check ☐ name ☐ 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy C Garrow_forwardC This question shows how molecular orbital (MO) theory can be used to understand the chemical properties of elemental oxygen O₂ and its anionic derivative superoxide Oz. a) Draw the MO energy diagram for both O2 and O2. Clearly label your diagram with atomic orbital names and molecular orbital symmetry labels and include electrons. Draw the Lewis structure of O2. How does the MO description of O2 differ from the Lewis structure, and how does this difference relate to the high reactivity and magnetic properties of oxygen? ) Use the MO diagram in (a) to explain the difference in bond length and bond energy between superoxide ion (Oz, 135 pm, 360 kJ/mol) and oxygen (O2, 120.8 pm, 494 kJ/mol).arrow_forwardPlease drawarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





