
Interpretation:
The reason for the puckered shape of cyclohexane and
Concept introduction:
Cyclohexane is a cyclic group of
The structure of cyclohexane is as follow:
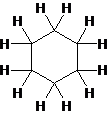
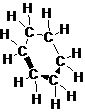
(side view)
To draw electron dot structure of trifluoromethyl methyl ether, you have to get aware about the valence electron of carbon, fluorine and oxygen.
The valence shall electron pair repulsion (VSEPR) theory is a simple and useful way to predict and rationalize the shape of molecule. The angle between atoms can also be find out.
| Bonding electron | Lone pair electrons | Shape | Angle |
| Linear | |||
| Trigon al planar | |||
| Bent | |||
| Tetrahedral | |||
| Trigon al pyramidal | |||
| Bent | |||
| Trigon al bi pyramidal | |||
| seesaw | |||
| t-shape | |||
| Linear | |||
| Octahedral | |||
| Square pyramidal | |||
| Square planer | |||
| Pentagonal bi pyramidal | |||
| Pentagonal pyramidal | |||
| Planar pentagonal |
To determine:
Explain why cyclohexane, a substance that contains six membered ring of carbon atoms is not flat but instead has a puckered, non-planar shape.
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
Chemistry, Books a la Carte Plus Mastering Chemistry with eText -- Access Card Package (7th Edition)
- In reactions whose kinetic equation is v = k[A]m, the rate coefficient k is always positive. Is this correct?arrow_forwardIf the concentration of A decreases exponentially with time, what is the rate equation? (A). -d[A] (B). dt d[A] = k[A] e-kt dtarrow_forwardGiven the first-order reaction: aA → products. State its kinetic equation.arrow_forward
- The following chemical structure represents a molecule of what molecular formula?arrow_forwardWhich region(s) of the following phospholipid is/are hydrophobic? RO I hydro-water phobic-dislikes = Hydrophobic dislikes water ○ I only Il only I and III only II and IV only O II, III, and IV only III || IVarrow_forwardPredict the product of the following reactions: O 0= excess Х Кон ОН H+ H+ Iarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





