
Chemistry, Books a la Carte Plus Mastering Chemistry with eText -- Access Card Package (7th Edition)
7th Edition
ISBN: 9780133900811
Author: John E. McMurry, Robert C. Fay, Jill Kirsten Robinson
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 8, Problem 8.32CP
(a)
Interpretation Introduction
To determine:
The geometry around the central atom in given model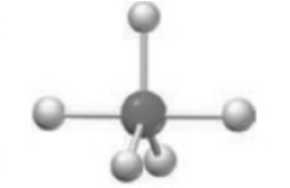
(b)
Interpretation Introduction
To determine:
The geometry around the central atom in given model:
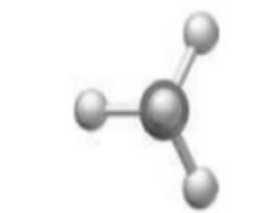
(c)
Interpretation Introduction
To determine:
The geometry around the central atom in given model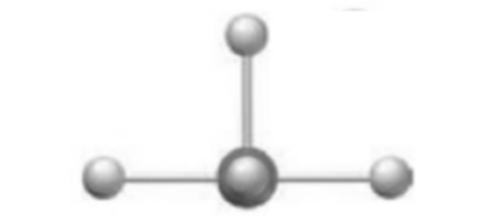
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
1. Calculate the accurate monoisotopic mass (using all 1H, 12C, 14N, 160 and 35CI) for your product using the table in
your lab manual. Don't include the Cl, since you should only have [M+H]*. Compare this to the value you see on
the LC-MS printout. How much different are they?
2. There are four isotopic peaks for the [M+H]* ion at m/z 240, 241, 242 and 243. For one point of extra credit,
explain what each of these is and why they are present.
3. There is a fragment ion at m/z 184. For one point of extra credit, identify this fragment and confirm by
calculating the accurate monoisotopic mass.
4. The UV spectrum is also at the bottom of your printout. For one point of extra credit, look up the UV spectrum
of bupropion on Google Images and compare to your spectrum. Do they match? Cite your source.
5. For most of you, there will be a second chromatographic peak whose m/z is 74 (to a round number). For one
point of extra credit, see if you can identify this molecule as well and confirm by…
Please draw, not just describe!
can you draw each step on a piece of a paper please this is very confusing to me
Chapter 8 Solutions
Chemistry, Books a la Carte Plus Mastering Chemistry with eText -- Access Card Package (7th Edition)
Ch. 8 - Prob. 8.1PCh. 8 - Prob. 8.2ACh. 8 - PRACTICE 8.3 Acetic acid, CH3CO2H , is the main...Ch. 8 - APPLY 8.4 Benzene, C6H6 , is a cyclic molecule in...Ch. 8 - PRACTICE 8.5 Identify the orbitals that overlap to...Ch. 8 - APPLY 8.6 Describe the bonding in propane, C3H8 ,...Ch. 8 - PRACTICE 8.7 Describe the hybridization of the...Ch. 8 - APPLY 8.8 Describe the hybridization of each...Ch. 8 - PRACTICE 8.9 Describe the hybridization of the...Ch. 8 - APPLY 8.10 Describe the hybridization of the...
Ch. 8 - Prob. 8.11PCh. 8 - Conceptual APPLY 8.12 Match the following...Ch. 8 - Prob. 8.13PCh. 8 - Prob. 8.14ACh. 8 - Prob. 8.15PCh. 8 - Prob. 8.16ACh. 8 - Prob. 8.17PCh. 8 - Prob. 8.18ACh. 8 - Prob. 8.19PCh. 8 - Prob. 8.20ACh. 8 - Prob. 8.21PCh. 8 - Prob. 8.22ACh. 8 - PRACTICE 8.23 Draw two resonance structures for...Ch. 8 - APPLY 8.24 Draw two resonance structures for the...Ch. 8 - Prob. 8.25PCh. 8 - Prob. 8.26PCh. 8 - PROBLEM 8.27 Identify which of the following...Ch. 8 - Prob. 8.28PCh. 8 - Prob. 8.29PCh. 8 - Prob. 8.30PCh. 8 - Prob. 8.31CPCh. 8 - Prob. 8.32CPCh. 8 - Prob. 8.33CPCh. 8 - Prob. 8.34CPCh. 8 - Prob. 8.35CPCh. 8 - Prob. 8.36CPCh. 8 - Prob. 8.37CPCh. 8 - Prob. 8.38CPCh. 8 - Prob. 8.39CPCh. 8 - Prob. 8.40CPCh. 8 - Two dichioroethylene molecules with the same...Ch. 8 - Prob. 8.42SPCh. 8 - Prob. 8.43SPCh. 8 - Prob. 8.44SPCh. 8 - How many charge clouds are there around the...Ch. 8 - Prob. 8.46SPCh. 8 - Prob. 8.47SPCh. 8 - What shape do you expect for each of the following...Ch. 8 - Prob. 8.49SPCh. 8 - Prob. 8.50SPCh. 8 - Prob. 8.51SPCh. 8 - Prob. 8.52SPCh. 8 - Prob. 8.53SPCh. 8 - Acrylonitrile is used as the starting material for...Ch. 8 - Predict values for all bond angles in dimethyl...Ch. 8 - Oceanographers study the mixing of water masses by...Ch. 8 - Prob. 8.57SPCh. 8 - Prob. 8.58SPCh. 8 - Prob. 8.59SPCh. 8 - Prob. 8.60SPCh. 8 - Prob. 8.61SPCh. 8 - Prob. 8.62SPCh. 8 - Prob. 8.63SPCh. 8 - Prob. 8.64SPCh. 8 - Prob. 8.65SPCh. 8 - Oxaloacetic acid is an intermediate involved in...Ch. 8 - The atoms in the amino acid glycine are connected...Ch. 8 - Prob. 8.68SPCh. 8 - Prob. 8.69SPCh. 8 - Prob. 8.70SPCh. 8 - 8.71 What is the difference between London...Ch. 8 - 8.72 What are the most important kinds of...Ch. 8 - Of the substances Xe,CH3Cl,HF, which has: (a) The...Ch. 8 - 8.74 Methanol boils nearlyhigher than methane, but...Ch. 8 - Prob. 8.75SPCh. 8 - Prob. 8.76SPCh. 8 - Prob. 8.77SPCh. 8 - Prob. 8.78SPCh. 8 - Prob. 8.79SPCh. 8 - Prob. 8.80SPCh. 8 - 8.81 Draw three-dimensional structures of PCl3 and...Ch. 8 - Prob. 8.82SPCh. 8 - Prob. 8.83SPCh. 8 - Prob. 8.84SPCh. 8 - Prob. 8.85SPCh. 8 - 8.86 A liquid sample contains methylamine (CH3NH2)...Ch. 8 - Prob. 8.87SPCh. 8 - Prob. 8.88SPCh. 8 - Prob. 8.89SPCh. 8 - Prob. 8.90SPCh. 8 - Prob. 8.91SPCh. 8 - Prob. 8.92SPCh. 8 - Prob. 8.93SPCh. 8 - Prob. 8.94SPCh. 8 - Prob. 8.95SPCh. 8 - Prob. 8.96SPCh. 8 - Prob. 8.98CPCh. 8 - Prob. 8.99CPCh. 8 - Prob. 8.100CPCh. 8 - Prob. 8.101CPCh. 8 - Prob. 8.102CPCh. 8 - Prob. 8.103CPCh. 8 - Prob. 8.104CPCh. 8 - Prob. 8.105CPCh. 8 - Prob. 8.106CPCh. 8 - Prob. 8.107CPCh. 8 - Prob. 8.108CPCh. 8 - Prob. 8.109CPCh. 8 - The odor of cinnamon oil is due to cinnamaldehyde,...Ch. 8 - Prob. 8.111CPCh. 8 - Prob. 8.112CPCh. 8 - Prob. 8.113CPCh. 8 - Prob. 8.114CPCh. 8 - Prob. 8.115CPCh. 8 - Prob. 8.116CPCh. 8 - Prob. 8.117CPCh. 8 - Prob. 8.118CPCh. 8 - Prob. 8.119MPCh. 8 - Prob. 8.120MPCh. 8 - Prob. 8.121MP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- > Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? esc ? A O O •If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. • If your answer is no, check the box under the drawing area instead. olo 18 Ar Explanation Check BB Click and drag to start drawing a structure. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accessibilityarrow_forwardName the structurearrow_forward> For each pair of substrates below, choose the one that will react faster in a substitution reaction, assuming that: 1. the rate of substitution doesn't depend on nucleophile concentration and 2. the products are a roughly 50/50 mixture of enantiomers. Substrate A Substrate B Faster Rate X CI (Choose one) (Choose one) CI Br Explanation Check Br (Choose one) C 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy A F10arrow_forward
- How to draw this mechanism for the foloowing reaction in the foto. thank youarrow_forwardPredict the major products of the following organic reaction: Some important notes: CN A? • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. No reaction. Explanation Check Click and drag to start drawing a structure. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Centerarrow_forwardDraw the major product of the following reaction. Do not draw inorganic byproducts. H3PO4 OHarrow_forward
- Predict the major products of this organic reaction: HBr (1 equiv) Δ ? Some important notes: • Draw the major product, or products, of this reaction in the drawing area below. • You can draw the products in any arrangement you like. • Pay careful attention to the reaction conditions, and only include the major products. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. • Note that there is only 1 equivalent of HBr reactant, so you need not consider the case of multiple additions. Explanation Check X ©2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacyarrow_forwardFor the structure below, draw the resonance structure that is indicated by the curved arrow(s). Be sure to include formal charges. :ÖH Modify the second structure given to draw the new resonance structure. Include lone pairs and charges in your structure. Use the + and - tools to add/remove charges to an atom, and use the single bond tool to add/remove double bonds.arrow_forwardUsing the table of Reactants and Products provided in the Hints section, provide the major product (with the correct stereochemistry when applicable) for questions below by selecting the letter that corresponds to the exact chemical structures for the possible product. OH conc Hydrochloric acid 40°C Temp A/arrow_forward
- Using arrows to designate the flow of electrons, complete the reaction below and provide a detailed mechanism for the formation of the product OH conc Hydrochloric acid 40°C Temp All chemical structures should be hand drawn on a piece of paper Paragraph BI UAE +varrow_forwarddraw out the following structures plesearrow_forwardDraw everything on a piece of paper outlining the synthesis from acetaldehyde to 2 cyclopentene carboxaldehyde using carbon based reagants with 3 carbons or fewers. Here is the attached image.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY