
Concept explainers
Interpretation:
The pH values after the addition of each proportion of the base to the acid is to be determined. Also, the titration curve needs to be drawn.
Concept introduction:
Titration curve is drawn to determine the change in pH of an acid or base with respect to the added volume of base or acid to it.
The titration curve can be drawn between a strong/weak acid and strong/weak base. The change in pH shows different patterns for different combinations of acids and bases.
Answer to Problem 69E
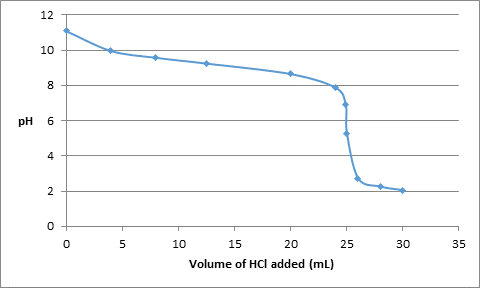
The change in pH due to addition of HCl is represented as follows:
| Volume of HCl added (mL) | pH |
| 0 | 11.1 |
| 4.0 | 9.97 |
| 8.0 | 9.58 |
| 12.5 | 9.25 |
| 20.0 | 8.65 |
| 24.0 | 7.87 |
| 24.9 | 6.9 |
| 25.0 | 5.28 |
| 26.0 | 2.71 |
| 28.0 | 2.24 |
| 30.0 | 2.04 |
The titration curve can be drawn as follows:
Explanation of Solution
Initial pH of the analyte solution; Ammonia is a weak base that forms an equilibrium when dissolved in water. The equilibrium is as follows.
The molarity of ammonia at 0 mL HCl is 0.100 M. The ICE table for its dissociation can be represented as follows:
| Reaction | Ammonia | Ammonium | OH- |
| Initial | 0.1 | 0 | 0 |
| Change | -x | +x | +x |
| Equilibrium | (0.1-x) | x | x |
The value of x can be calculated by solving the equation as follows:
Then the pH of the initial solution can be determined.
Thus, pH of solution when 0 mL of acid is added is 11.1.
Addition of
When 4.0 mL of acid is added the number of moles of ammonia and HCl can be calculated from their molarity and volume as follows:
The ICE table can be represented as follows:
| Reaction | Ammonia | HCl | Ammonium chloride |
| Initial | 0.0025 | 0.0004 | 0 |
| Change | -0.0004 | -0.0004 | +0.0004 |
| Equilibrium | 0.0021 | 0 | 0.0004 |
The pH can be calculated using the Henderson-Hesselbalch equation as follows:
Now,
In the Henderson-Hasselbalch equation, the pKa is used. therefore, the pKa for ammonia need to be calculated using its pKb.
Applying the Henderson-Hasselbalch equation,
Thus, the pH of the solution is 9.97.
Addition of
Total amount of ammonia to be neutralized
Amount of acid added
Then the ICE table after the addition of base is created in order to determine the pH of the solution using Henderson-Hasselbalch equation.
| Reaction | Ammonia | HCl | Ammonium chloride |
| Initial | 0.0025 | 0.0008 | 0 |
| Change | -0.0008 | -0.0008 | +0.0008 |
| Equilibrium | 0.0017 | 0 | 0.0008 |
Applying the Henderson-Hasselbalch equation,
Addition of
Total amount of ammonia to be neutralized
Amount of acid added
Then the ICE table after the addition of base is created in order to determine the pH of the solution using Henderson-Hasselbalch equation.
| Reaction | Ammonia | H+ | Ammonium chloride |
| Initial | 0.0025 | 0 | 0 |
| Change | -0.00125 | -0.00125 | +0.00125 |
| Equilibrium | 0.00125 | 0 | 0.00125 |
Applying the Henderson-Hasselbalch equation,
Addition of
Total amount of ammonia to be neutralized
Amount of acid added
Then the ICE table after the addition of base is created in order to determine the pH of the solution using Henderson-Hasselbalch equation.
| Reaction | Ammonia | H+ | Ammonium |
| Initial | 0.0025 | 0 | 0 |
| Change | -0.002 | -0.002 | +0.002 |
| Equilibrium | 0.0005 | 0 | 0.002 |
Applying the Henderson-Hasselbalch equation,
Addition of
Total amount of ammonia to be neutralized
Amount of acid added
Then the ICE table after the addition of base is created in order to determine the pH of the solution using Henderson-Hasselbalch equation.
| Reaction | Ammonia | HCl | Ammonium chloride |
| Initial | 0.0025 | 0 | 0 |
| Change | -0.0024 | -0.0024 | 0.0024 |
| Equilibrium | 0.0001 | 0 | 0.0024 |
Applying the Henderson-Hasselbalch equation,
Addition of
Total amount of ammonia to be neutralized
Amount of acid added
Then the ICE table after the addition of base is created in order to determine the pH of the solution using Henderson-Hasselbalch equation.
| Reaction | Ammonia | HCl | Ammonium chloride |
| Initial | 0.0025 | 0 | 0 |
| Change | -0.00249 | -0.00249 | -0.00249 |
| Equilibrium | 0.00001 | 0 | 0.00249 |
Applying the Henderson-Hasselbalch equation,
Addition of
Total amount of ammonia to be neutralized
Amount of acid added
Then the ICE table after the addition of base is created in order to determine the pH of the solution using Henderson-Hasselbalch equation.
| Reaction | Ammonia | H+ | Ammonium |
| Initial | 0.0025 | 0 | 0 |
| Change | -0.0025 | -0.0025 | -0.0025 |
| Equilibrium | 0.0000 | 0 | 0.0025 |
From the number of moles of ammonium ion and total volume that is 0.05 L, the molarity of ammonium chloride solution can be calculated as follows:
The ammonium ion from salt can react with water to give back ammonia as follows:
The ICE table can be prepared as follows:
The acid dissociation constant can be represented as follow:
From the ICE table,
Since, the value of acid dissociation constant is very low thus; value of x can be neglected from denominator.
This is the concentration of hydrogen ion in the solution.
The pH can be calculated as follows:
Addition of 26 mL of the acid:
The number of moles of ammonia and acid will be:
The reaction is represented as follows:
| Reaction | Ammonia | HCl | Ammonium choride |
| Initial | 0.0025 | 0.0026 | 0 |
| Change | -0.0025 | -0.0025 | +0.0025 |
| Equilibrium | 0 | 0.0001 | 0.0025 |
The pH depends only on the concentration of HCl as it is a strong acid.
The molarity of HCl using total volume of the solution will be:
The pH can be calculated as follows:
Addition of 28 mL of acid:
The number of moles of each species will be:
The reaction is represented as follows:
| Reaction | Ammonia | HCl | Ammonium choride |
| Initial | 0.0025 | 0.0028 | 0 |
| Change | -0.0025 | -0.0025 | +0.0025 |
| Equilibrium | 0 | 0.0003 | 0.0025 |
The pH depends only on the concentration of HCl as it is a strong acid.
The molarity of HCl using total volume of the solution will be:
The pH can be calculated as follows:
Addition of
The number of moles of ammonia and HCl can be calculated as follows:
Then the ICE table after the addition of base is created in order to determine the pH of the solution using Henderson-Hasselbalch equation.
| Reaction | Ammonia | H+ | Ammonium | OH- |
| Initial | 0.0025 | 0.003 | 0 | 0 |
| Change | -0.0025 | -0.0025 | 0 | 0 |
| Equilibrium | 0 | 0.0005 | 0 | 0 |
Concentration of hydrogen ion
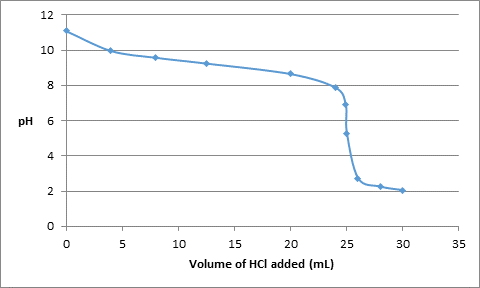
The data obtained from the above calculations will be:
| Volume of HCl added (mL) | pH |
| 0 | 11.1 |
| 4.0 | 9.97 |
| 8.0 | 9.58 |
| 12.5 | 9.25 |
| 20.0 | 8.65 |
| 24.0 | 7.87 |
| 24.9 | 6.9 |
| 25.0 | 5.28 |
| 26.0 | 2.71 |
| 28.0 | 2.24 |
| 30.0 | 2.04 |
The titration curve can be drawn as follows:
Want to see more full solutions like this?
Chapter 8 Solutions
EBK CHEMICAL PRINCIPLES
- Please help me Please use https://app.molview.com/ to draw this. I tried, but I couldn't figure out how to do it.arrow_forwardPropose a synthesis of 1-butanamine from the following: (a) a chloroalkane of three carbons (b) a chloroalkane of four carbonsarrow_forwardSelect the stronger base from each pair of compounds. (a) H₂CNH₂ or EtzN (b) CI or NH2 NH2 (c) .Q or EtzN (d) or (e) N or (f) H or Harrow_forward
- 4. Provide a clear arrow-pushing mechanism for each of the following reactions. Do not skip proton transfers, do not combine steps, and make sure your arrows are clear enough to be interpreted without ambiguity. a. 2. 1. LDA 3. H3O+ HOarrow_forwardb. H3C CH3 H3O+ ✓ H OHarrow_forward2. Provide reagents/conditions to accomplish the following syntheses. More than one step is required in some cases. a. CH3arrow_forward
- Identify and provide an explanation that distinguishes a qualitative and quantitative chemical analysis. Provide examples.arrow_forwardIdentify and provide an explanation of the operational principles behind a Atomic Absorption Spectrometer (AAS). List the steps involved.arrow_forwardInstructions: Complete the questions in the space provided. Show all your work 1. You are trying to determine the rate law expression for a reaction that you are completing at 25°C. You measure the initial reaction rate and the starting concentrations of the reactions for 4 trials. BrO³¯ (aq) + 5Br¯ (aq) + 6H* (aq) → 3Br₂ (l) + 3H2O (l) Initial rate Trial [BrO3] [H*] [Br] (mol/L) (mol/L) | (mol/L) (mol/L.s) 1 0.10 0.10 0.10 8.0 2 0.20 0.10 0.10 16 3 0.10 0.20 0.10 16 4 0.10 0.10 0.20 32 a. Based on the above data what is the rate law expression? b. Solve for the value of k (make sure to include proper units) 2. The proposed reaction mechanism is as follows: i. ii. BrО¸¯ (aq) + H+ (aq) → HBrO3 (aq) HBrO³ (aq) + H* (aq) → H₂BrO3* (aq) iii. H₂BrO³* (aq) + Br¯ (aq) → Br₂O₂ (aq) + H2O (l) [Fast] [Medium] [Slow] iv. Br₂O₂ (aq) + 4H*(aq) + 4Br(aq) → 3Br₂ (l) + H2O (l) [Fast] Evaluate the validity of this proposed reaction. Justify your answer.arrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





